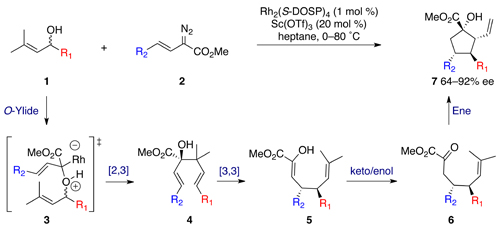
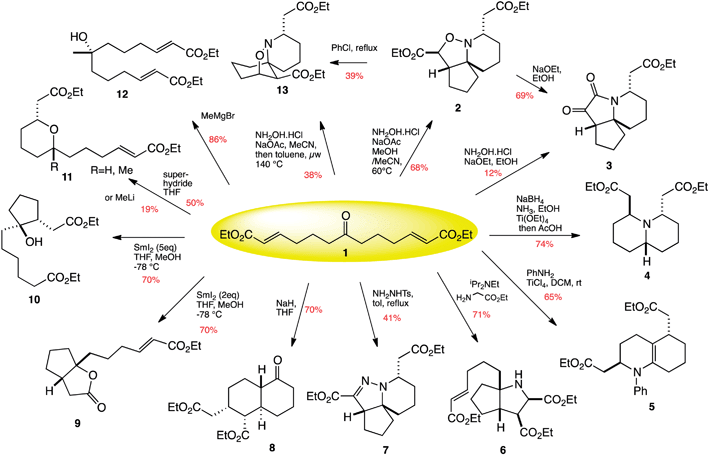
In July I met up with Clyde Hutchison (J. Craig Venter Institute) at ISACS5 in Manchester, UK. He gave a great talk at the meeting and afterwards I caught up with him to find out more about his career. A short excerpt from the interview is copied below but you can read the full version in Chemistry World.
_180_tcm18-206964.jpg) Clyde Hutchison is a distinguished professor at the J. Craig Venter Institute, San Diego, US, and is also Kenan Professor Emeritus at the University of North Carolina, Chapel Hill. His research focuses on the search for improved methods to learn about gene function from DNA sequence information.
Clyde Hutchison is a distinguished professor at the J. Craig Venter Institute, San Diego, US, and is also Kenan Professor Emeritus at the University of North Carolina, Chapel Hill. His research focuses on the search for improved methods to learn about gene function from DNA sequence information.
Why did you decide to become a scientist?
My father was a chemist. He called himself a chemical physicist. He worked on paramagnetic resonance absorption problems and did some really major work in that area. He always encouraged me to learn about science. As a physical scientist, he had a tendency to think of biology as a bit on the messy side. I think in the end, though, he came to like what I did.
You did an undergraduate degree in physics. How did you make the transition to synthetic biology?
I knew I wanted to be a scientist but I didn’t know I wanted to be a biologist. I was also considering a maths major but it came down to office hours. At Yale there was a particular day that you had to declare your major field of study and before you did so, you had to go and speak to the advisor in that field. The physics advisor’s office hours ended later than the maths advisor’s so that’s why I chose physics.
To find out how Professor Hutchison ended up being a biologist, read the full interview.
For more information about ISACS5, check out my conference blog.











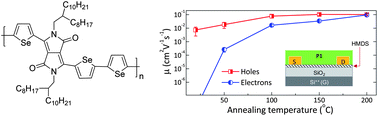
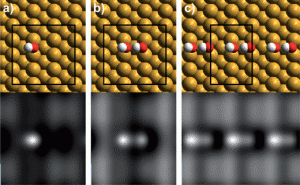
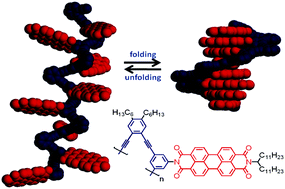 While the influence of π-π interactions on systems which fold into highly ordered structures, or foldamers, has been previously studied, the system designed by Würthner and his team is unique in that the rigid OPE backbone played no part in directing the folding geometry; π-π interactions in the PBI units were the sole influence on the backbone conformation and lead to the final geometry.
While the influence of π-π interactions on systems which fold into highly ordered structures, or foldamers, has been previously studied, the system designed by Würthner and his team is unique in that the rigid OPE backbone played no part in directing the folding geometry; π-π interactions in the PBI units were the sole influence on the backbone conformation and lead to the final geometry.