 US chemists have devised an efficient synthesis of a natural product with great potential as a protectant against chemical warfare agents and in the treatment of Alzheimer’s disease.
US chemists have devised an efficient synthesis of a natural product with great potential as a protectant against chemical warfare agents and in the treatment of Alzheimer’s disease.
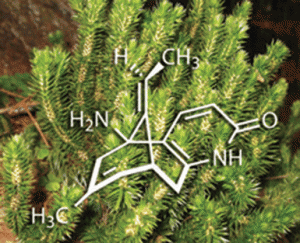
Huperzia serrata, one of an ancient lineage of plants known as the firmosses, has been much in demand lately because it contains a chemical known as (-)-huperzine A. This alkaloid is a potent and selective inhibitor of acetylcholine esterase, and as a result is able to counteract the action of certain chemical warfare agents, such as sarin and VX. There are also strong suggestions that it may slow the progression of neurological diseases such as Alzheimer’s disease. A team of organic chemists led by Seth Herzon at Yale University, New Haven, has now developed a high-yielding route to this elusive natural product, opening up opportunities for its wider clinical evaluation.
‘The primary obstacle to the clinical development of (-)-huperzine A has been one of supply,’ says Herzon. He points out that the average yield from the dried herb is just 0.011 per cent, a problem compounded by the nearly 20 years it takes to reach maturity, coupled with its increasing scarcity due to overharvesting in its native China. To address these problems, several groups have in the past devised syntheses of (-)-huperzine A, with the best to date employing 16 steps and giving an overall yield of about 2.8 per cent. Herzon and his team have now beaten this by a factor of 16, with an eight-step synthesis that gives 25-45 per cent overall yield.
Read the full story in Chemistry World and download Herzon’s Chemical Science Edge article.










