In this Chemical Science Edge article, researchers from the Center for Research in Biological Chemistry and Molecular Materials (CIQUS) and Department of Organic Chemistry at the University of Santiago de Compostela, describe a highly elegant method of controlling and changing the helical sense and elongation of poly(phenylacetylene)s. This is achieved via modifying the polymer backbone with a chiral group which responds to different solvent environments in a reliable way. The system seems ideal for chemical sensor technology development.
The polymer highlighted in the article is a poly(phenylacetylene) derivatised with a chiral methoxytrifluoro-phenylacetic acid (MTPA) group. The monomer was prepared from the alkyne 4-ethynylaniline, (R)-α-methoxy-α-(trifluoromethyl)phenylacetic acid and oxalyl chloride in two steps. Subsequent polymerization was carried out using a rhodium norbornadiene dimer catalyst, under argon, and the polymer was precipitated from a tetrahydrofuran (THF) solution using methanol and hexane.
The key to the effects described in changes to the polymer properties is due to the chemical structure of the pendant group, which contains an amide and a chiral centre, giving rise to four stereoisomers. The amide group function can result in cis or trans geometry, and the carbonyl and chiral centre yield syn (sp) or anti-periplanar (ap) conformers.
In a range of solvents, the resultant 4 states of this moiety have profound and different effects on the elongation and the sense (or direction), and tightness (compression or elongation), of the helical polymer. The effect is shown here:
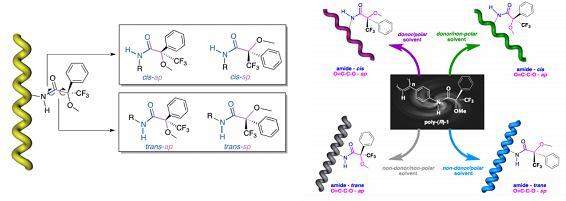
Solvent donor effects which destabilise the amide group to a cis orientation were proven by UV-Vis spectroscopy to elongate and change the sense of the helicate. A bathochromic shift was observed. The authors suggest that solvent polarity plays a greater role with the carbonyl-methoxy group in changing the sense (or direction) of the helicate, via circular dichroism measurements. Overall, any destabilisation in the pendant group is accomodated in the polymer backbone, via a change in the orientation and elongation of the helicate, resulting in a new stable state.
Sequential stimulation of the two functional groups was performed, as were experiments using thin films of the polymer. A large amount of analytical data: IR, NMR, UV-Vis, Raman spectroscopy and differential scanning calorimetry (DSC) is presented, as are atomic force microscopy (AFM) measurements and findings from molecular mechanics calculations.
This work should prove of interest to polymer chemists and sensor researchers alike, and to the wider scientific community.
Controlled Modulation of the Helical Sense and Elongation of Poly(phenylacetylene)s by Polar and Donor Effects
Ricardo Riguera, Felix Freire, Seila Leiras, José Manuel Seco and Emilio Quiñoá
Chem. Sci., 2013, Accepted Manuscript
DOI: 10.1039/C3SC50835H
Kevin Murnaghan is a guest web-writer for Chemical Science. He is currently a Research Chemist in the Adhesive Technologies Business Sector of Henkel AG & Co. KGaA, based in Düsseldorf, Germany. His research interests focus primarily on enabling chemistries and technologies for next generation adhesives and surface treatments. Any views expressed here are his personal ones and not those of Henkel AG & Co. KGaA.
Comments Off on A chiral, responsive handle on poly(phenylacetylene) properties














-429---NS_tcm18-221866.jpg)


-429---NS_tcm18-221958.jpg)



