Haw Yang is Associate Professor of Chemistry and Director of Graduate Studies at Princeton University, USA. Haw and his experimental physical chemistry group work on single-molecule chemical dynamics and develop new single-molecule- and single-nanoparticle-based methods for the study of complex systems. His team’s ultimate goal is to arrive at a level of understanding that affords a quantitative prediction of the dynamics and how they contribute to systems behaviour.
Haw Yang is Associate Professor of Chemistry and Director of Graduate Studies at Princeton University, USA. Haw and his experimental physical chemistry group work on single-molecule chemical dynamics and develop new single-molecule- and single-nanoparticle-based methods for the study of complex systems. His team’s ultimate goal is to arrive at a level of understanding that affords a quantitative prediction of the dynamics and how they contribute to systems behaviour.
Haw serves as one of Chemical Science’s Associate Editors, handling submissions in physical chemistry.
Who or what inspired you to study physical chemistry?
Madame Curie. I really didn’t know who she was until, as a kid, I watched a movie about her, based on her daughter’s autobiography. That movie left a very strong impression on me because I saw her dedication and her passion for science. And she did it, not for personal gain, but for humankind – for years, she used her discovery to cure cancer. She went from doing fundamental research to helping humanity, and that left an enduring mark on me.
She was also very good at maths and physics and chemistry, and – because I’m a geek – this impressed me enormously, which is another reason why she became my heroine.
For you, what is the biggest and most important unanswered question in the chemical sciences?
The quantitative prediction of complex systems behaviour from first-principles understanding of atomistic and molecular actions.
Outside of science, what would your dream job be?
As I said, I’ve been a nerd, a geek, since I was a kid – I’ve never been any good at anything else. So, if I hadn’t been able to get a job in this profession, I would have been doomed! (Laughs)
So this – what I’m doing now – is my dream job. If I had to do it all again, I would do it exactly the same way – I would come to Princeton, and do what I’m doing right now. People pay me to do what I enjoy doing; not everyone can have this kind of career! I feel extremely lucky. This is the dream.
What do you consider the most fulfilling part of your job?
To see my students and post-docs do extremely well, do something really creative after they leave my group – and to have them still remember me! (Laughs) That’s really awesome. Sometimes I get emails or postcards from former students out of the blue, and I’d say, wow, I did not expect this.

“For me, the cello is a fitting instrument – it would let me be alone and be quiet, and at the same time, do something creative” – Haw Yang (Image © Shutterstock)
Making new discoveries in the lab – I don’t often get to do that anymore, but when I was still in the lab, I knew I was the first in the world to see those results and interpret them – that was extremely fulfilling. And these days, I take joy in doing experiments that people have been telling me are impossible to do. (Laughs) I love a challenge, I love to change the way people think.
Which musical instrument do you, or wish you could, play?
I wish that I could learn to play cello – it’s on my to-do list when I get the time. I guess I’m hitting that age when I’d like something more quiet, more introspective, and the cello has that quality, especially if one can play Bach’s unaccompanied cello concertos – those are my favourites. In this line of work, we’re alone most of the time, and we think a lot – that is, if we’re lucky, we get time to think. So for me, the cello is a fitting instrument – it would let me be alone and be quiet, and at the same time, do something creative.
Fire, earth, water, or air?
Water.
Describe Chemical Science in three words.
Breaking status quo
Your personal message to Chem Sci authors and readers?
What always gets me excited is original physical chemistry, broadly defined. It could be a new and relevant physical chemistry problem that’s well articulated, an ingenious approach that definitely answers an outstanding question, an innovative technique that enables new experiments to solve problems that matter, or a conceptual breakthrough that inspires new thinking.
We scientists are in the business of breaking the status quo, and we should do so with high scientific rigour. I strongly believe scientists must never do work which merely repeats what is already known, for the sake of hype, of joining the bandwagon, or aiming to please.
So, if you agree with me that ground-breaking substance and genuine originality are more important than hype, I invite you to submit your most creative papers to Chemical Science – your work could help define the future of physical chemistry!
Haw Yang and our dynamic international team of Associate Editors make direct decisions on the content of Chemical Science and actively drive its scientific development – submit your best and most innovative work to any of their Editorial Offices.
Read Haw Yang’s latest article in Chemical Science:
Harnessing thermal fluctuations for purposeful activities: the manipulation of single micro-swimmers by adaptive photon nudging
Bian Qian, Daniel Montiel, Andreas Bregulla, Frank Cichos and Haw Yang
Chem. Sci., 2013,4, 1420-1429
DOI: 10.1039/C2SC21263C, Edge Article
Our Associate Editors Haw Yang, Kopin Liu and Kazunari Domen have selected their recommended physical chemistry papers on Chemical Science – read their Editors’ Choice selection today and find out why they think these are must-reads!













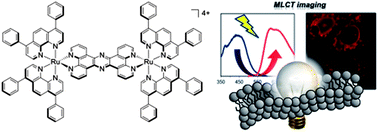
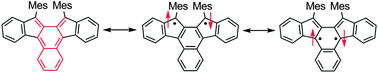

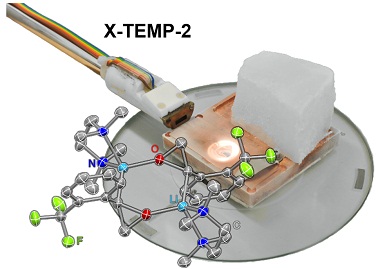
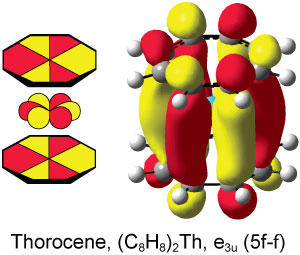


 Scientists from Japan have
Scientists from Japan have