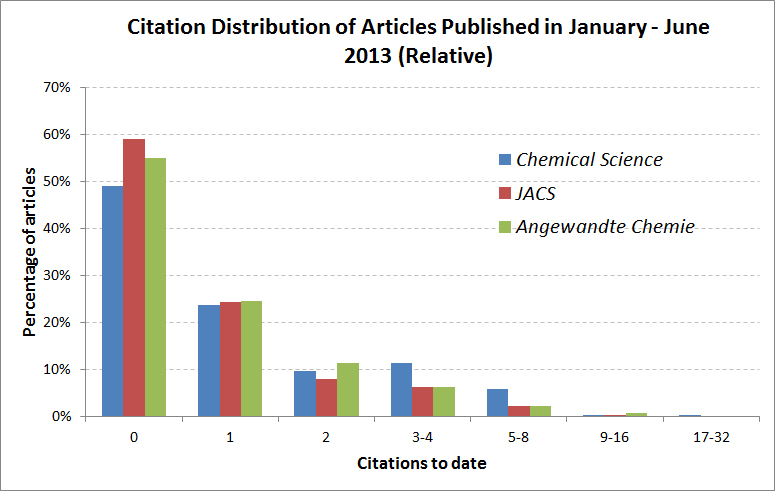
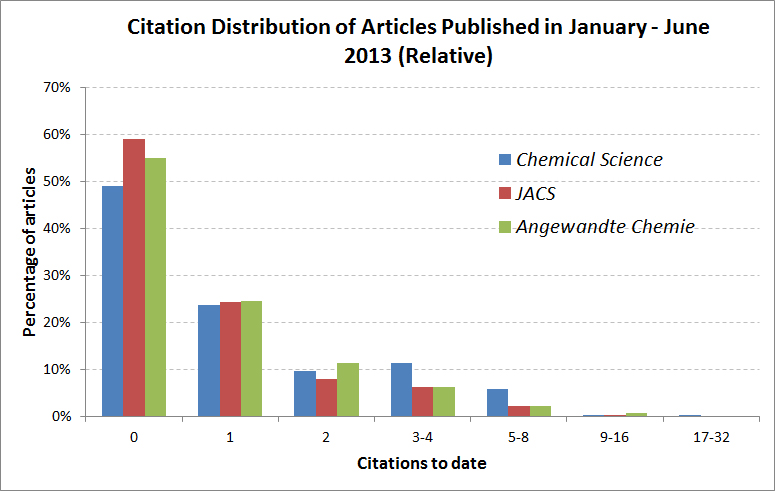
The 10 most accessed Chemical Science articles between July and September 2013 were as follows:
Intramolecular C(sp3)–H amination
Jenna L. Jeffrey and Richmond Sarpong
Chem. Sci., 2013,4, 4092-4106
DOI: 10.1039/c3sc51420j
Pd(ii)-catalyzed alkoxylation of unactivated C(sp3)–H and C(sp2)–H bonds using a removable directing group: efficient synthesis of alkyl ethers
Fa-Jie Chen, Sheng Zhao, Fang Hu, Kai Chen, Qi Zhang, Shuo-Qing Zhang and Bing-Feng Shi
Chem. Sci., 2013,4, 4187-4192
DOI: 10.1039/c3sc51993g
1,2,3-Triazoles as versatile directing group for selective sp2 and sp3 C–H activation: cyclization vs substitution
Xiaohan Ye, Zhengrong He, Tonia Ahmed, Keith Weise, Novruz G. Akhmedov, Jeffrey L. Petersen and Xiaodong Shi
Chem. Sci., 2013,4, 3712-3716
DOI: 10.1039/c3sc51211h
Benzofuran synthesis via copper-mediated oxidative annulation of phenols and unactivated internal alkynes
Ruyi Zhu, Jiangbo Wei and Zhangjie Shi
Chem. Sci., 2013,4, 3706-3711
DOI: 10.1039/c3sc51489g
Pd(ii)-catalyzed alkylation of unactivated C(sp3)–H bonds: efficient synthesis of optically active unnatural a-amino acids
Kai Chen, Fang Hu, Shuo-Qing Zhang and Bing-Feng Shi
Chem. Sci., 2013,4, 3906-3911
DOI: 10.1039/c3sc51747k
Palladium-catalyzed heteroallylation of unactivated alkenes – synthesis of citalopram
Joanne F. M. Hewitt, Lewis Williams, Pooja Aggarwal, Craig D. Smith and David J. France
Chem. Sci., 2013,4, 3538-3543
DOI: 10.1039/c3sc51222c
Gold(i)-catalyzed enantioselective bromocyclization reactions of allenes
Dillon H. Miles, Marcos Veguillas and F. Dean Toste
Chem. Sci., 2013,4, 3427-3431
DOI: 10.1039/c3sc50811k
Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide
David S. Surry and Stephen L. Buchwald
Chem. Sci., 2011,2, 27-50
DOI: 10.1039/c0sc00331j
“Phosphite–urea” cooperative high-turnover catalysts for the highly selective bromocyclization of homogeranylarenes
Yasuhiro Sawamura, Hidefumi Nakatsuji, Akira Sakakura and Kazuaki Ishihara
Chem. Sci., 2013,4, 4181-4186
DOI: 10.1039/c3sc51432c
Prediction of suitable catalyst by 1H NMR: asymmetric synthesis of multisubstituted biaryls by chiral phosphoric acid catalyzed asymmetric bromination
Keiji Mori, Yuki Ichikawa, Manato Kobayashi, Yukihiro Shibata, Masahiro Yamanaka and Takahiko Akiyama
Chem. Sci., 2013,4, 4235-4239
DOI: 10.1039/c3sc52142g
If you have any thoughts or comments on any of these articles, why not leave these in the comment box below?
Fancy submitting your own work to to Chemical Science? You can submit online today or alternatively you can email us your suggestions.











 Tom Muir is the Van Zandt Williams Jr. Class of ’65 Professor of Chemistry at Princeton University, USA.
Tom Muir is the Van Zandt Williams Jr. Class of ’65 Professor of Chemistry at Princeton University, USA.