Fluorescent imaging is one of the most useful techniques in modern day medicine, as it provides us with a method of visualizing the inside of a body to allow for diagnosis and treatment of disease. Typically, an organic fluorophore is attached to a biomolecule that, upon introduction to the body, is able to interact with a specific type of molecule in the body, such as a cancerous cell or a molecule that carries out a particular and important function. More recently, in addition to the use of biomolecules, fluorescent labeling has turned to the polymer world for applications in targeted drug delivery or cell patterning to name but a few examples. Fluorescence becomes important in these applications, as the introduction of non-biological molecules to the body requires a method of locating them to track and monitor their activity.
There are a number of methods of introducing organic fluorophores to polymers, for example, polymerization of fluorescently labeled monomer units, end-group attachment, or post-synthetic modification, all of which offer advantages and disadvantages. The one factor that all of these approaches have in common however, is that one needs to beware of how the attachment of fluorophores, which are typically large, will change the chemistry of the polymer. It would therefore be advantageous if small, yet fluorescent, groups could be attached to polymers without otherwise changing their properties.
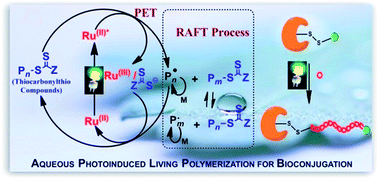
It is this interest in synthesising fluorescent polymers using small molecules that Mathew Robin and Rachel O’Reilly from the University of Warwick sought to tackle. They were able to demonstrate that the introduction of dithiomaleimide functional groups, which have a large Stokes shift (250 nm) and bright emission, to acrylate or methacrylate polymers did not change the properties of the polymer itself. Perhaps even more interestingly is that it was demonstrated that the functional groups could be introduced both pre-synthetically and post-synthetically. In this way, polymer fluorescence could be both turned on in a profluorescent polymer that contained a reactive dibromomaleimide monomer unit, as well as reversibly turned off through a dithiol exchange reaction to a non-fluorescent dithiomaleimide monomer unit.
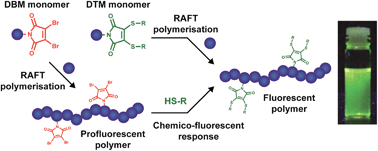
The development of a relatively simple system whose fluorescence can be reversible turned on and off is an exciting step forward in developing polymers, especially since the end groups of this functionalised polymer allows for its further incorporation into more complex polymeric systems. These polymers could have applications in not only the biomedical uses discussed, but also in a numerous other applications such as organic electronic devices, sensing materials and polymer materials like nanoparticles and hydrogels.
Read this HOT Chemical Science Edge article in full for free*!
Fluorescent and chemico-fluorescent responsive polymers from dithiomaleimide and dibromomaleimide functional monomers
Mathew P. Robin and Rachel K. O’Reilly
Chem. Sci., 2014, 5, 2717.
DOI: 10.1039/C4SC00753K, Edge Article

About the Writer
Anthea Blackburn is a guest web writer for Chemical Science. Anthea is a graduate student hailing from New Zealand, studying at Northwestern University in the US under the tutelage of Prof. Fraser Stoddart (a Scot), where she is exploiting supramolecular chemistry to develop multidimensional systems and study the emergent properties that arise in these superstructures. When time and money allow, she is ambitiously attempting to visit all 50 US states before graduation.
*Access is free untill 08.08.14 through a registered RSC account – click here to register
Comments Off on Lighting Up the Polymer World, Reversibly