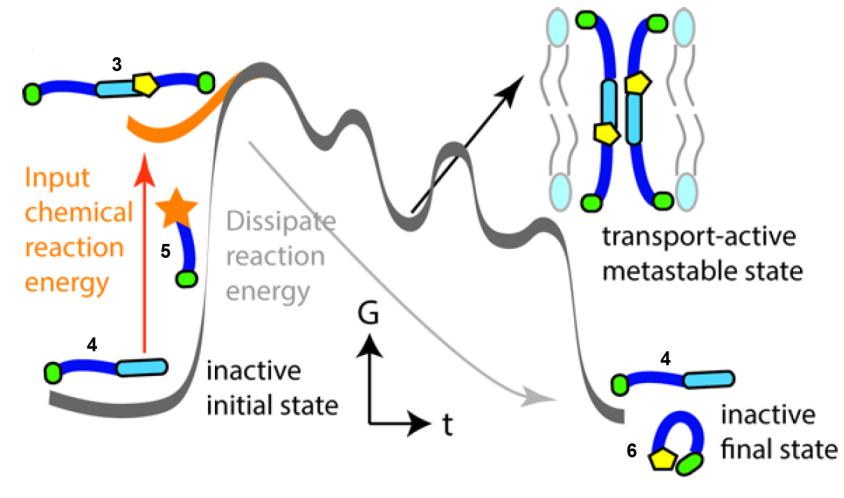
In nature, many cellular processes are controlled by structures that are only assembled when they are needed, in response to a chemical energy input. The structure (and hence the action of the structure) is maintained so long as the chemical energy input, or ‘fuel’ for the process is present. For example, microtubules can ‘grow’ or ‘shrink’ depending on the presence of guanosine triphosphate (GTP), and hence GTP serves as a ‘fuel’ for the function of the microtubules.
Inspired by this principle, Tom Fyles and his team from University of Victoria, Canada have used thiol–thioester exchange chemistry to assemble a metastable ion channel that disassembles over time in the absence of a chemical fuel.
In the scheme below, thiol 4 is not an active ion channel, but the addition of thioester 5 causes the formation of 3 by thiol-thioester exchange. 3 forms an active ion channel, but is metastable and therefore over time it disassembles into 4 + 6 which stops the ion transport. Adding more 5 (the fuel) will temporarily regenerate the ion transport as more C is formed.
While this system functions exactly as planned, its design was not without complications, says Fyles: “One setback was that these compounds do not work in vesicles so we had to develop reaction monitoring techniques using the bilayer clamp experiment. That gives us exquisite sensitivity, but also allows us to detect very minor amounts of impurities. So another setback was the observation that an oxidation impurity was vastly more active than the compounds we wanted to see. This problem was easy enough to solve, but it took some time to recognize what was going on.”
This work also opens up exciting new avenues of investigation for the future for Fyles and his team. “The more general insight is that this system depends entirely on kinetic factors. As chemists we are used to thinking about rates in terms of the reaction order and uniform reactant concentrations in the bulk. Our system additionally shows the importance of local concentrations as opposed to the bulk concentration. This introduces additional kinetic components related to diffusion. The functioning of our system recorded using the bilayer clamp is largely controlled by diffusion processes, rather than the reaction kinetics we control in the design of the system. This suggests that we might be able to make other systems that deliberately exploit non-uniform concentrations – yet another feature we can see in nature. Stay tuned.”
To download the full article for free*, click the link below:
Dissipative Assembly of a Membrane Transport System
A. K. Dambenieks, P. H. Q. Vu and T. M. Fyles
DOI: 10.1039/C4SC01258E
*Access is free untill the 17.07.14 through a registered RSC account – click here to register