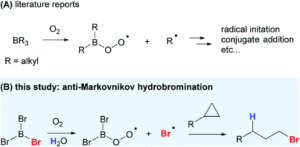
Redox-active transition metal complexes, those that can undergo multiple oxidation and reduction events, are ideal candidates for electrochemical energy storage and fuel technologies. A significant caveat to employing these complexes for electrochemical processes is that their solubilities can drastically change across redox states, creating insoluble oxidation or reduction products that precipitate out of solution. Even when the insoluble redox product is still chemically-intact (as in a reversible electrochemical reaction), it can often be difficult to electrochemically convert it back to its original, soluble redox state. Researchers in the US have now come up with a new technique to overcome this, using ferrocene as a redox mediator to assist with electron-transfer between the insoluble materials and electrodes.
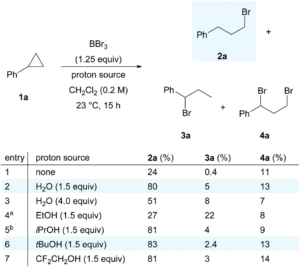
The researchers studied the redox-active nickel complex, [Ni(PPh2NPh2)2(CH3CN)]2+, which is often used as a catalyst for electrochemical hydrogen evolution. The electrochemistry of the nickel complex was explored under non-catalytic conditions, where the absence of a proton source was previously unexplored. Cyclic voltammetry experiments of [Ni(PPh2NPh2)2]2+ in acetonitrile indicated two, one-electron reduction events that correspond to the NiII/Iand NiI/0 redox couples, both of which were electrochemically and chemically reversible at low concentrations (Figure 1). The researchers noted a concentration dependence, where the reversibility is increasingly lost at higher concentrations of the complex (blue scan, Figure 1A). This was attributed to the formation of the two-electron reduced product, [Ni(PPh2NPh2)2], which proved insoluble in acetonitrile, precipitating out of solution upon its electrochemical formation and depositing on the electrode surface.
![Cyclic voltammograms of [Ni(PPh2NPh2)2]2+ in acetonitrile](https://blogs.rsc.org/sc/files/2020/11/CS-Fig-1-184x300.gif)
Figure 1. Cyclic voltammograms of [Ni(PPh2NPh2)2]2+, showing the two redox events with the two, separate peaks. A) Concentration dependence, whereby reversibility decreases upon increasing concentration and B) Scan-rate dependence, whereby reversibility is regained at higher scan-rates.
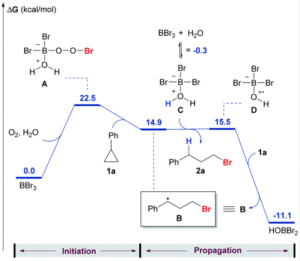
![Redox cycle scheme for [Ni(PPh2NPh2)2]2+](https://blogs.rsc.org/sc/files/2020/11/CS-Fig-2-300x123.gif)
Figure 2. A scheme showing the redox cycle of [Ni(PPh2NPh2)2]2+, with annotations to describe the experimental kinetics observed.
To find out more, please read:
Katherine J. Lee, Kunal M. Lodaya, Cole T. Gruninger, Eric S. Rountree and Jillian L. Dempsey
Chem. Sci., 2020, 11, 9836-9851
About the blogger:
 Dr. Samantha Apps just finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps just finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.















 Established in 1920, the School of Chemistry and Chemical Engineering of Nanjing University is one of the world’s most active and recognised chemistry institutions. After a century of development and evolution, it is now a globally leading department dedicated to advancing the chemical sciences through cutting-edge research and world-renowned education. Chemical Science and the Royal Society of Chemistry are delighted to help celebrate the 100th anniversary of chemical sciences in Nanjing University with a
Established in 1920, the School of Chemistry and Chemical Engineering of Nanjing University is one of the world’s most active and recognised chemistry institutions. After a century of development and evolution, it is now a globally leading department dedicated to advancing the chemical sciences through cutting-edge research and world-renowned education. Chemical Science and the Royal Society of Chemistry are delighted to help celebrate the 100th anniversary of chemical sciences in Nanjing University with a