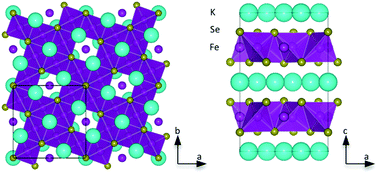
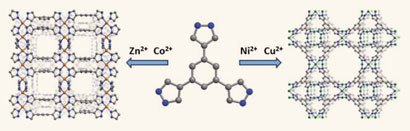
The most chemically and thermally stable metal-organic frameworks (MOFs) yet have been made by a team in the US. The MOFs could surpass zeolites as industrial catalysts.
Natural zeolites are porous alumina-silicate rocks used as catalysts in industrial processes. However, their pore sizes and surface functionalisations are difficult to alter, which limits their performance. MOFs – made by joining up metal oxide clusters with linking organic molecules – have similar structures to zeolites and are therefore of interest as alternatives. Until now, they have not been robust enough to withstand the conditions that zeolites undergo during industrial processes. Traditionally, MOFs have only been stable in temperatures up to 500 degrees Celsius, have low chemical stability and some even fall apart in water.
Jeffrey Long from the University of California, Berkeley, and colleagues have made MOFs that can withstand temperatures of 510 degrees Celsius and a pH range of 2 to 14. They made the MOFs by reacting a metal salt, such as nickel chloride or nickel nitrate, with trispyrazolylbenzene. The organic molecules were deprotonated and the functional groups were bound to the metal to create a three-dimensional network.
Find out more by reading the full news story in Chemistry World and downloading Professor Long’s Chemical Science Edge article.
Also of interest:
Modifying MOFs: new chemistry, new materials
Seth M. Cohen, Chem. Sci., 2010, 1, 32-36
Hydrogen storage and carbon dioxide capture in an iron-based sodalite-type metal–organic framework (Fe-BTT) discovered via high-throughput methods
Kenji Sumida, Satoshi Horike, Steven S. Kaye, Zoey R. Herm, Wendy L. Queen, Craig M. Brown, Fernande Grandjean, Gary J. Long, Anne Dailly and Jeffrey R. Long, Chem. Sci., 2010, 1, 184-191











 Scientists have laid the foundations for a high-performance ‘molecular fridge’ capable of reaching temperatures within a few thousandths of a degree of absolute zero (0K) with a high degree of efficiency. Such ultracoolers could have applications in areas such as ultra-low temperature physics, where alternative technologies such as those that rely on expensive and rare helium-3 could be unsuitable or too costly.
Scientists have laid the foundations for a high-performance ‘molecular fridge’ capable of reaching temperatures within a few thousandths of a degree of absolute zero (0K) with a high degree of efficiency. Such ultracoolers could have applications in areas such as ultra-low temperature physics, where alternative technologies such as those that rely on expensive and rare helium-3 could be unsuitable or too costly.