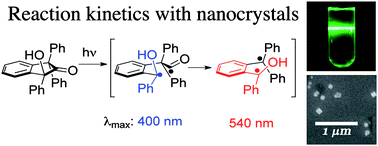
Phthalocyanines (Pcs) have excellent electronic and optical properties, which make them promising as a photosensitisers (PSs) for photodynamic therapy (PDT). Since Pcs strongly and selectively absorb light in the range of 600-800nm, they allow high penetration depth of light in normal tissue while minimising the risk and complications such as burning. But Pcs are highly hydrophobic and tend to form aggregates in aqueous media, which reduces their therapeutic activity.
Various types of nanocarriers, such as micelles, liposomes and nanoparticles, have been used to overcome this problem, and to prepare a stable dispersion of Pc in aqueous solution. However, most of them still suffer from shortcomings such as poor loading of Pc (small weight % of Pc in nanocarriers), risk of payload leaking before reaching target cells, and laborious, time-consuming fabrication and encapsulation processes.
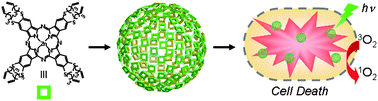
Scientists in the Republic of Korea have made ZnPc nanospheres in one pot without using any templates or emulsifiers. They can be post-synthetically modified to improve their dispersibility in aqueous solution without altering their morphology or properties. They show higher singlet oxygen generation efficiency and in vitro phototoxicity than monomeric Pc molecules, suggesting that they are potentially useful as a photosensitiser for photodynamic therapy.
Targeting ligands could be introduced to deliver the nanospheres to specific target sites, anticipate the researchers. And if therapeutic agents were encapsulated, they could perform dual chemo- and photodynamic therapy.
Read this ‘HOT’ Chemical Science article:
Self-assembled, covalently linked, hollow phthalocyanine nanospheres
Raghunandan Hota, Kangkyun Baek, Gyeongwon Yun, Youngkook Kim, Hyuntae Jung, Kyeng Min Park, Eunjin Yoon, Taiha Joo, Juseok Kang, Chan Gyung Park, Su Mi Bae, Woong Shick Ahn and Kimoon Kim
Chem. Sci., 2012, DOI: 10.1039/C2SC21254D












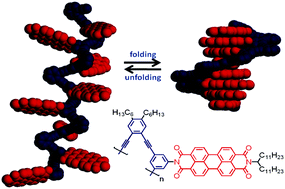 While the influence of π-π interactions on systems which fold into highly ordered structures, or foldamers, has been previously studied, the system designed by Würthner and his team is unique in that the rigid OPE backbone played no part in directing the folding geometry; π-π interactions in the PBI units were the sole influence on the backbone conformation and lead to the final geometry.
While the influence of π-π interactions on systems which fold into highly ordered structures, or foldamers, has been previously studied, the system designed by Würthner and his team is unique in that the rigid OPE backbone played no part in directing the folding geometry; π-π interactions in the PBI units were the sole influence on the backbone conformation and lead to the final geometry.  As with many natural products, purifying one from a suite of similar compounds can be tricky. But
As with many natural products, purifying one from a suite of similar compounds can be tricky. But