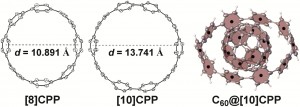
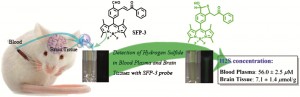
US scientists have developed a pH sensor based on nanocrystal quantum dots designed to be used in a biological pH range. pH is an important factor in monitoring tumour health and the efficacy of anticancer treatments, and the sensor could be injected into tumours to monitor their health in real time.
Nanocrystal-based pH sensors have been reported before as they have attractive properties, but the sensors operate in alkali conditions, making them unsuitable for biological applications. To overcome this problem, Daniel Nocera from the Massachusetts Institute of Technology, Cambridge, and colleagues, tailored their sensor so that it could operate at pHs between 6 and 8 (physiological pH).
Read the full article in Chemistry World
Link to journal article
A Nanocrystal-based Ratiometric pH Sensor for Natural pH Ranges
R C Somers et al
Chem. Sci., 2012, DOI: 10.1039/c2sc20212c