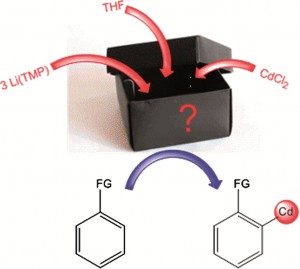
Metallation is an entry point to constructing compounds, and by converting inert C-H bonds to reactive C-metal bonds, it opens up bond forming opportunities. Mixed metal reagents are widely used; combining an alkali metal with a softer, less reactive metal charges the softer metal component with super-reactivity, which, combined with good selectivity and functional group tolerances, makes the reagents superior to organolithium reagents. But how do they work?
To remove the mystery surrounding these mixed-metal reagents, scientists in the UK have studied one such reagent in detail. They discover (surprisingly) that LiCd(TMP)3 is unlikely to be an ate as previously thought, instead consisting of two independent homometallic amides. Rather than a synergistic metallation of the substrate, the metallation is a two step process: ortholithiation followed by transmetallation to cadmium.
Link to journal article
Opening the black box of mixed-metal TMP metallating reagents: direct cadmation or lithium-cadmium transmetallation
D R Armstrong et al
Chem. Sci. , 2012, DOI: 10.1039/c2sc20392h