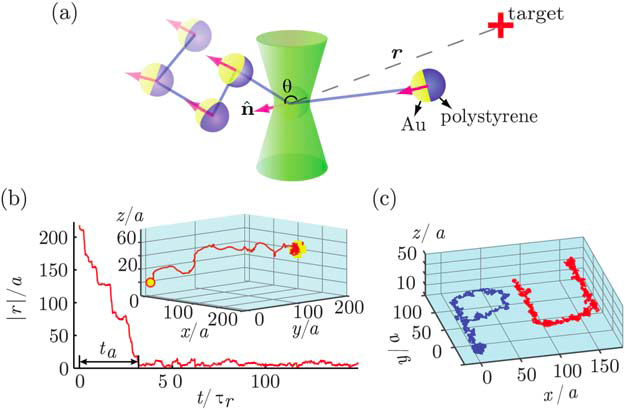
In a recent Edge article, Chemical Science Associate Editor Professor Haw Yang and his group at Princeton University use Brownian motion to transport a two-sided micro-particle to a target, with the help of ‘photon-nudging’ to keep it on track.
The micrometer precision of this simple method of locomotion is notable. It could potentially be used to deliver drugs and molecules in cells, precisely and in real-time.
Normally, Brownian motion causes micro-particles to move randomly in fluids. This presents a challenge for the creation of controllable ‘micro-swimmers’. Yet, with some external direction, this motion can deliver particles to their targets.
Yang and co-workers ‘steer’ a two-sided microsphere in a fluid, as it undergoes Brownian motion. One side has a gold coating, whilst the other is polystyrene. When the polystyrene side is facing the target, a laser beam is activated. The light then moves this ‘Janus’ particle in one of two ways, either by ‘radiation-pressure’ (pushing by photons) or by heat-induced locomotion via the photophoretic effect. The latter effect forces this particle, when it has a large temperature difference across its two faces, to move in the direction of the cooler side (polystyrene side) (see also Crooke’s radiometer).
The authors do not use ‘photon-nudging’ constantly. It is used occasionally, just to steer the particle in the right direction. Brownian motion does most of the work transporting the particle to the target– making this a good example of a man-made Brownian motor.
It will be interesting to see how soon researchers can refine this ‘photon-nudging’ method to achieve nanometer or molecular scale accuracy. Many would welcome that level of control for particles and molecules in cells and chemical solutions.
Read this HOT Chem Sci cover article in full!
Harnessing thermal fluctuations for purposeful activities: the manipulation of single micro-swimmers by adaptive photon nudging
Bian Qian, Daniel Montiel, Andreas Bregulla, Frank Cichos and Haw Yang
Chem. Sci., 2013, 4, 1420-1429
DOI: 10.1039/C2SC21263C
Geoff Nelson is a guest web-writer for Chemical Science. He currently works as a post-doctoral research associate in Dr David Payne’s research group in the Department of Materials at Imperial College, London. Geoff’s current research concerns the synthesis and characterization of post-transition metal oxides for use in the energy sector. His other research interests include carbon-based materials, biophysical chemistry, and surface science.













-429---NS_tcm18-221958.jpg)