Geoff Nelson, our new guest web-writer for Chemical Science, blogs about recent findings on van der Waals interactions in non-polar liquids. Read his first Chem Sci blog post below:
Professor Christopher Hunter, in his latest Edge article, notes that the thermodynamic properties of the van der Waals interactions between non-polar molecules can be predicted based on their calculated molecular surface areas (0.3 kJ mol-1 Å-2). His findings help simplify computational approaches to the design of molecular binding sites or self-assembled molecules.
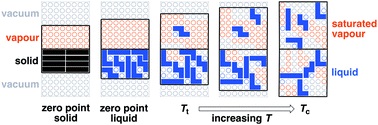
Prof Hunter’s article includes a detailed model for the behaviour of the van der Waals interaction at liquid-vapor and liquid-liquid interfaces. Each molecule has ‘surface contact points’ capable of van der Waals interactions with the external environment. These contacts can be made and broken, depending on the space around the molecule. The total number of contacts determines the total van der Waals contribution to free energy. This model helps explain the physical basis of several thermodynamic events (e.g., melting).
The choice of non-polar liquids as the chemical system to study was ideal to isolate the van der Waals interaction, as other non-covalent interactions are minimised (e.g., electrostatic).
Potent pharmaceuticals and stable self-assembled structures depend on effective binding between molecules. Predicting the chemical structure necessary to promote such binding is now made easier by Professor Hunter’s research.
Read this Chemical Science Edge article in full:
van der Waals interactions in non-polar liquids
Christopher A. Hunter
Chem. Sci., 2013,4, 834-848
DOI: 10.1039/C2SC21666C
Geoff Nelson is a new guest web-writer for Chemical Science. He currently works as a post-doctoral research associate in Dr David Payne’s research group in the Department of Materials at Imperial College, London. Geoff’s current research concerns the synthesis and characterization of post-transition metal oxides for use in the energy sector. His other research interests include carbon-based materials, biophysical chemistry, and surface science.