Ever wanted to find a way to replace a carbon-carbon (CC) double bond with another bond that will change the physical and chemical properties of a molecule without significantly altering its sterics? Look no further than a boron-nitrogen bond! BN/CC isosterism involves substituting a CC double bond with a BN bond, which can substantially change the electronic properties of a molecule while keeping it the same size. This isosterism could be a powerful tool in biomedical studies of biologically relevant arene-containing organic molecules, which are plentiful. However, few studies report on the differences in functions cause by substituting a BN bond into an arene. Initial results suggest that the BN compounds can have similar or increased activity and availability when compared to the natural, all carbon molecules.
Researchers in the United States synthesized a BN-analogue of tryptophan (Figure 1) for use as an unnatural amino acid (UAA) to study and intentionally alter the properties of proteins. Tryptophan, in addition to making Americans sleepy at Thanksgiving, is relatively rare, but participates in pi system interactions and is the primary source of native protein fluorescence. This makes it an important target for UAA research. The researchers synthesized the sodium salt of BN-tryptophan in a 6-step process, which can be modified to resolve the two enantiomers by chiral HPLC. The BN-tryptophan exhibits noticeably red-shifted absorbance and emission spectra, with the fluorescence maximum shifted by almost 40 nm in the BN compound.
In order to test whether the BN-tryptophan could be incorporated into proteins, researchers incorporated it into media without tryptophan and monitored whether E. coli cells that lacked the ability to produce tryptophan would grow. They found that the cells grew when in the presence of BN-tryptophan, but to a significantly lesser degree than with an equivalent quantity of natural tryptophan. However, cell growth increased when the media contained both BN-tryptophan and natural tryptophan. This suggests that cells will accept BN-tryptophan as a tryptophan analogue, but they don’t tolerate full replacement well.
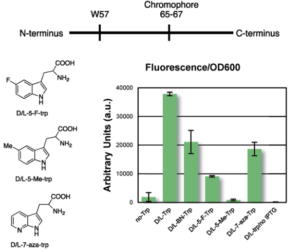
Figure 2. Representation of the protein sequence, structures of other tryptophan analogues, and fluorescence plot for the studied substrates.
Further studies incorporated BN-tryptophan and three other previously utilized tryptophan analogues into a green fluorescent protein (GFP). For fluorescence to be detected, the analogue must be incorporated into the protein and then accurately read by cells. The BN-tryptophan performs as well or better than the established tryptophan analogues, proving its functionality (Figure 2). The proteins with BN-tryptophan also demonstrate several different properties than those containing natural tryptophan; their fluorescence is red shifted and they are more susceptible to oxidation by hydrogen peroxide. These alterations in activity could prove useful in future studies.
To find out more please read:
Katherine Boknevitz, James S. Italia, Bo Li, Abhishek Chatterjee and Shih-Yuan Liu
Chem. Sci., 2019, 10, 4994–4998.
About the blogger:
Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.