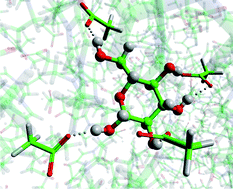
The precise mechanism for the dissolution of cellulose by ionic liquids is hotly debated, with some researchers insisting that the ionic liquid cation forms a hydrogen bond to the sugar’s OH groups, without data to back it up.
Now researchers have proved conclusively with experimental data that there are no hydrogen bonding interactions between the cation and the sugars.
Read Christopher Hardacre’s Chemical Science Edge article to find out more.
Also of interest:
How polar are ionic liquids? Solutions of charge-transfer salts in ionic liquids reveal a dual nature of solvent polarity and an absence of ion pairing










