Why do biological enzymes exhibit superior catalytic activity? Well, this is mainly because they can firmly anchor reactants into catalytically active pockets using their three-dimensional structures. Inspired by this nano-confinement effect, scientists have developed “nanozymes,” a family of artificial enzymes.
A group of scientists from the University of New South Wales (Australia), University of Utah (USA), and Ruhr-Universität Bochum (Germany) investigated the interplay between the degree of nano-confinement and oxygen reduction reaction (ORR) activity. They discovered that ORR activity only scaled proportionally to the degree of confinement at low overpotentials (ORR theoretical potential: 1.23 V vs. RHE). The results have been published in Chemical Science (doi: 10.1039/C9SC05611D).
Synthesized by etching Ni from Pt-Ni alloy nanoparticles, metallic Pt nanoparticles possessing channels of different opening sizes served as the nanozymes with different confinement degrees. When the Ni content increased, the channel of the etched nanoparticles widened. Specifically, Pt nanozymes prepared from Pt-Ni nanoparticles with Pt/Ni = 1/1.5 (NZsmall) contained 69% channel openings smaller than 2 nm (Fig. 1a); Whereas those from the precursors with Pt/Ni = 1/2.5 (NZmedium) and 1/3 (NZlarge) had 52% (Fig. 1b) and 34% (Fig. 1c) <2-nm-wide channels, respectively. Therefore, the nano-confinement degrees of the three nanozymes followed the sequence of NZsmall>NZmedium>NZlarge.
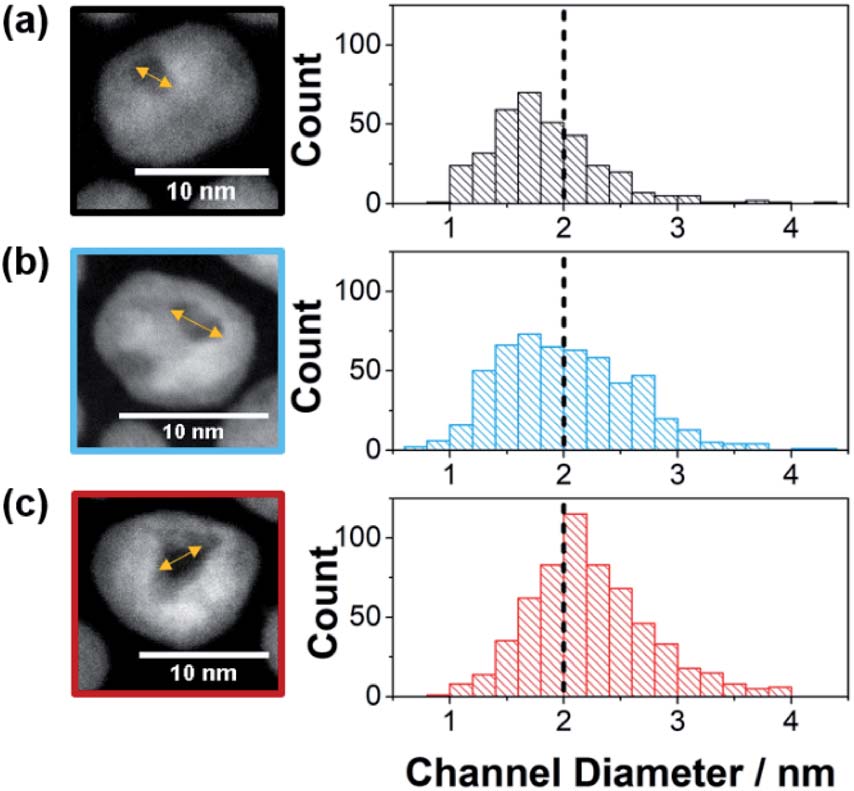
Figure 1. High-resolution transmission electron microscopy images (left) and channel diameter distribution profiles (right) of (a) NZsmall, (b) NZmedium, and (c) NZlarge.
The ORR activity of the three nanozymes strongly depended on the magnitude of the overpotential. With the outer surfaces passivated by surfactants, the ORR activities of the nanozymes were only associated with O2 reduction within their channels. At low overpotentials (Fig. 2, inset), NZsmall had the highest ORR activity among all the catalysts evaluated by the authors, as indicated by its lowest kinetic current. At high overpotentials or low applied biases; however, the ORR activity of NZmedium and NZlarge rapidly increased (Fig. 2). NZmedium and NZlarge became more active than NZsmall at potentials lower than 0.82 V (vs. RHE) and 0.80 V (vs. RHE), respectively.
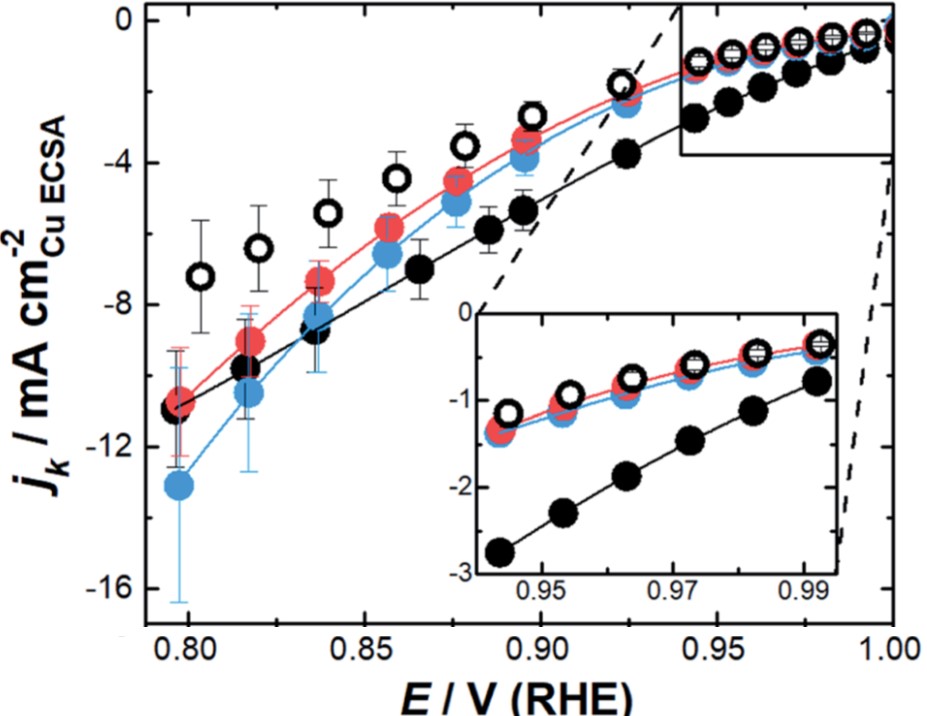
Figure 2. The potential (E)-dependence of kinetic current density (jk). Inset: low-overpotential (high potentials) region. Legends: solid black – NZsmall; solid blue – NZmedium; solid red – NZlarge; open black – mesoporous Pt nanoparticles without nano-channels. The consistently small absolute jk of the mesoporous nanoparticles reflected its relatively low ORR activity.
The finite element simulation revealed the underlying mechanism of the experimental results. At low overpotentials, the ORR activity was governed by the kinetics of O2-reduction. Due to high charge density, the local proton concentration inside the channels of NZsmall was the highest, leading to the fastest reaction and the highest ORR activity. At high overpotentials, the ORR activity became mass-transport limited. Nanozymes with wide channel openings, that is, NZmedium and NZlarge, allowed a large amount of O2 to diffuse into the channels, which enhanced O2 supply and augmented ORR activity.
This work unveiled the potential-dependence of the ORR catalytic activities of porous Pt nanoparticles under different degrees of nano-confinement. This insight could rationalize and further enable the design of nanozymes with tailorable ORR activities.
To find out more, please read:
The Importance of Nanoscale Confinement to Electrocatalytic Performance
Johanna Wordsworth, Tania M. Benedetti, Ali Alinezhad, Richard D. Tilley, Martin A. Edwards, Wolfgang Schuhmann, and J. Justin Gooding
Chem. Sci., 2019, DOI: 10.1039/C9SC05611D
Tianyu Liu acknowledges Zac Croft at Virginia Tech, U.S., for his careful proofreading of this post.
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from the University of California, Santa Cruz, in the United States. He is passionate about the communication of scientific endeavors to both the general public and other scientists with diverse research expertise to introduce cutting-edge research to broad audiences. He is a blog writer for Chem. Comm. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from the University of California, Santa Cruz, in the United States. He is passionate about the communication of scientific endeavors to both the general public and other scientists with diverse research expertise to introduce cutting-edge research to broad audiences. He is a blog writer for Chem. Comm. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










