Scientists from India and Denmark have found a way to go one better than x-ray crystallography to examine pharmaceutical crystals at an even deeper level. Their method could be used to distinguish between polymorphs – different crystal forms – of a compound to aid in drug design.
The team, led by Upadrasta Ramamurty and Gautam Desiraju from the Indian Institute of Science, Bangalore, and Andrew Bond from the University of Southern Denmark, have used nanoindentation to analyse two different polymorphs of aspirin. Polymorphs are crystals of the same compound but with a different molecular arrangement. Although two crystals may appear similar in structure, they can have dramatically different properties, and many drugs only receive regulatory approval for one form. ‘One of the current areas of research is trying to link crystal properties to crystal structure and to try to understand how polymorphism occurs,’ says Bond.
The nanoidentation technique involves depressing a nano-sized tip into the crystal. The researchers then measured the imprint left in the sample to determine the material’s mechanical properties, such as plasticity and elasticity (how easily a substance is deformed permanently and non-permanently, respectively).
The team discovered that two polymorph crystals of aspirin, which appeared to be pure by x-ray crystallography, in fact contained a mixture of the polymorph types. Nanoindentation could have an impact on the pharmaceutical industry, which currently relies on x-ray crystallography to establish whether or not a new drug has been made, for intellectual property rights.
Read the full story in Chemistry World and find out more by downloading the Chemical Science Edge article.












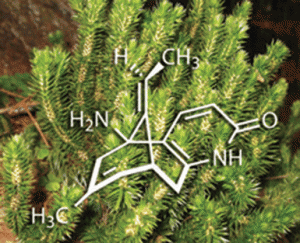
 As with many natural products, purifying one from a suite of similar compounds can be tricky. But
As with many natural products, purifying one from a suite of similar compounds can be tricky. But