US chemists have developed a new highly stereoselective route to a natural product with potent anticancer properties.
David MacMillan and colleagues at Princeton University made the structurally challenging diazonamide A by exploiting three different types of catalysis – Lewis acid, transition metal and organocatalysis – in the key steps.
The principal challenge was stereoselectively installing the C(10) quaternary carbon stereocentre, explains MacMillan, as this aspect of the structure had not been successfully addressed in any of the three completed total syntheses. Using asymmetric iminium catalysis, the team efficiently synthesised the furanindoline core and C(10) centre with high stereoselectivity, which they believe is the most complex and challenging setting in which organocatalysis has been employed to date.
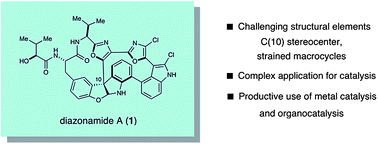
Find out more by downloading MacMillan’s Chemical Science Edge article.










