Metal nanoparticles that can catalyse organic reactions in water can be made using polyelectrolyte nanoreactors, claim scientists.
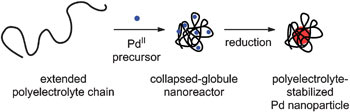
Thanks to their ionisable functional groups, polyelectrolytes can change their conformation in water. When there are few other ions in the solution, the chains stretch out because the charged groups on the chains repel each other.
But when Vy Dong, at the University of Toronto, Canada, and colleagues added an acidic palladium (II) chloride solution, the repulsive interactions were screened and the polyelectrolyte chains collapsed into globules around the chloride ions. Subsequent reduction of the palladium (II) ions in this collapsed-globule nanoreactor using sodium borohydride generated polyelectrolyte stabilised palladium nanoparticles that were bench stable for over a year.
The team used the nanoparticles as catalysts in aqueous Suzuki coupling reactions and achieved high yields at loadings as low as 0.01 mol % palladium. They now plan to transfer the counterion-induced collapse strategy to the synthesis of other polyelectrolyte–metal systems.
Read more about this work in Professor Dong’s Chemical Science Edge article.