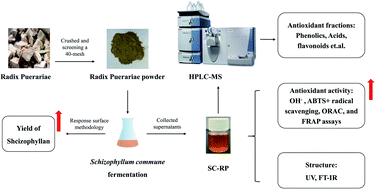
Evaluating total antioxidant capacity in food samples is essential for determining the antioxidant levels and managing oxidative stress-related health risks such as cardiovascular diseases, cancer, neurodegenerative diseases, diabetes, and inflammatory conditions. However, the current methods need complex equipment and procedures. Therefore, a simple, rapid and accurate method for detection is crucial. Work by Chen and co-workers addresses the same by introducing a copper-poly(tannic acid) (Cu-PTA) coordination polymer nanozyme synthesised through a formaldehyde-mediated sol–gel chemistry route that exhibits enhanced peroxidase-like catalytic activity, for sensitive and rapid colorimetric detection of total antioxidant capacity (TAC) in food samples such as fruit juices. The copper ions (Cu2+) coordinate with long-chain poly(tannic acid) molecules, yielding a three-dimensional network structure with substantial phenolic hydroxyl groups providing active sites for catalytic function. This structure creates a stable nanozyme with excellent water dispersibility and surface area. The nanozyme catalyses the oxidation of the chromogenic substrate 3,3’,5,5’-tetramethylbenzidine (TMB) which produces a strong visible blue colour change into oxidised form (oxTMB) in the presence of hydrogen peroxide (H2O2) which allows easy and colorimetric detection of enzyme activity. The introduction of antioxidants such as ascorbic acid inhibits this oxidation reaction by consuming reactive oxygen species generated in the catalytic cycle, thereby decreasing the colour intensity. The result is a fast, visually perceptible colorimetric assay where the blue colour intensity correlates inversely with antioxidant concentration. Using smartphone images analysed via red-green-blue (RGB) colour models, the assay enables on-site, simple TAC evaluation without bulky spectrophotometers. Further, the nanozyme follows Michaelis–Menten enzyme kinetics with higher affinities for TMB and H2O2 substrates compared to many natural peroxidases with improved catalytic performance.
Nanozymes like Cu-PTA overcome challenges like enzyme instability, high costs, and complex storage requirements that are usually seen with natural enzymes. This enhanced catalytic performance of nanomaterials with smartphone-based colorimetric detection adds to the emerging focus on the development of multiplexed and portable platforms for various applications such as disease diagnostics, nutritional monitoring, and pollution assessment. This comprehensive presentation of nanozyme kinetics and stability could aid with the development of portable sensor surface designs, integrating increased shelf-life of the sensors, enhanced signal amplification, and improved assay robustness. Additionally, the portable nature of the RGB-based readout resonates with integrating optics and image analysis into biomedical devices for real-time, quantitative health monitoring.
Read the article published in RSC Advances:
Yan Chen, Zhaohui Zhang, Yifan Ouyang, Aikebaier Reheman and Bei Jiang
RSC Adv., 2025,15, 11401-11408
About the Web Writer
Biography
Anna Anandita is a PhD candidate in Chemical Engineering at the Indian Institute of Technology, Jammu, India, specialising in nanozyme-enhanced smartphone-assisted microfluidics and biosensor development for healthcare applications. She completed her MTech and BTech degrees in Chemical Engineering, focusing on sensor design, environmental monitoring, and fluid transport phenomena. Anna collaborates on interdisciplinary projects integrating nanomaterial development, data science, machine learning, and transport modelling to develop innovative diagnostic devices. She is actively engaged in science communication, community outreach, and capacity-building initiatives to promote health equity and disaster resilience. You can connect with Anna through LinkedIn and social media platforms @thedumbbanshee.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest Popular Advances, Reviews, Collections & more by following us on BlueSky. You can also keep informed by signing up to our E-Alerts.