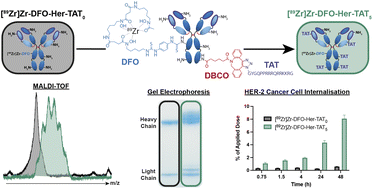
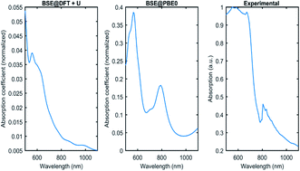
We are very pleased to introduce Professor Barry A. Blight, corresponding author on a recently accepted paper, ‘Colour tuneability of heteroleptic iridium complexes through second-sphere coordination’, which was well received by reviewers and was handpicked to be part of our Popular Advances collection.
Professor Blight told us more about his research group and the work that went into this study and what he hopes to achieve in the future. Read more of our 2024 Popular Advances collection here!
Gabrielle Bourguignon, Dr Mason Lawrence, Robert Horne, Ariane Volpé, Dr Barry Blight, Sarah Englehart, Aroosha Faheem, T. Harri Jones
The groups work focuses on the development of luminescent MOFs, investigating the properties if existing MOF materials and employing supramolecular techniques to explore organic optolectronic materials.
- Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
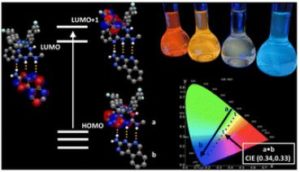
Iridium compounds are commonly applied as materials in Organic Light Emitting Diodes (OLEDs) due to their tuneable light emitting properties. We were investigating the potential to change the light emission properties of the compounds through inter-molecular communication with a complementary binder molecule.
- How big an impact could your results potentially have?
This study shows the potential to use communication between complementary designed emissive compounds to influence each other’s light emitting properties. It’s an interesting effect worth further consideration and it may have applications in future OLED development.
- Could you explain the motivation behind this study?
Our group works on all kinds of functional materials for a variety of applications. We were looking for ways to access difficult colours for OLED materials which led us to studying these a variety of this type of iridium compounds.
- In your opinion, what are the key design considerations for your study?
The key experimental design consideration was how to prove that the interaction between our iridium compound and the complimentary binder (through a host-guest interaction) was contributing to the change in colour. We had to use a variety of analytical and computational approaches to verify our hypothesis.
- Which part of the work towards this paper proved to be most challenging?
The computational work was most challenging. Our group traditionally doesn’t undertake computational studies. This was a new frontier for the team and one that came with a fair few challenges to overcome.
- What aspect of your work are you most excited about at the moment?
Each of the light emitting compounds in this work offer a unique property, some show the capacity to share energy, some show the ability to access difficult colours. We’re excited about the variety of applications these new iridium compounds may be used for.
- What is the next step? What work is planned?
Testing their applications and developing new and improved versions of the compounds, which may show further enhanced light emitting properties. We are particularly interested in modulating the materials for efficient white-light generation.
Read the article here:
Colour tuneability of heteroleptic iridium complexes through second-sphere coordination
We wish the group all the best with their future projects!
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest Popular Advances, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.