Argentina, Bolivia and Chile make up the “Lithium Triangle”, where 70 % of the world’s lithium reserves are concentrated. These factors lead to the study in the development of lithium batteries being of great interest to my country and particularly to me, because I am beginning to research on this topic, so I am interested in reading articles on lithium batteries. During the last decades, numerous investigations have been carried out in the area of lithium batteries, in relation to their components such as electrodes, separators and electrolytes, to make them safer, environmentally friendly and economical.
A battery is made up of a set of electrochemical cells, which are made up of three main components: cathode, anode and electrolyte. In the case of the lithium battery, when charging battery, the lithium ions move from the positive electrode (cathode) towards the negative electrode (anode), and in opposite way during the discharge process. In this type of battery, the negative electrode is mainly composed of carbonaceous materials and the positive electrode of metal oxides. There is an urgent need for better lithium-ion batteries in order to create sustainable solutions based on relatively limited resources with a long-term perspective, and to apply the knowledge of mesoporous based cathode materials to an exciting field of lithium-ion battery research.
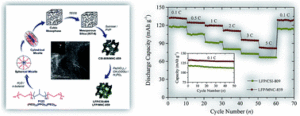
The article “Electrochemical performance of nano-sized LiFePO4-embedded 3D-cubic ordered mesoporous carbon and nitrogenous carbon composites” focuses on generic principles applicable to more advanced materials and systems for the development of highly electroactive olivine structured cathode materials for high performance lithium-ion batteries. This research findings would open potential avenues for fundamental on state-of-the-art mesoporous carbon-based materials for energy storage systems. Exploitation of novel porous cathode materials and a strengthening of fundamental understanding of their textural property and electrochemical performance relationships have played a major role in developing the lithium-ion battery research field.
One way to overcome the limitations of commercial lithium batteries is to modify the active material used in the negative electrode. Current devices use mainly carbonaceous material, which has the advantage of operating at low potentials. During the charging and discharging process, the carbonaceous material undergoes a reversible volumetric variation of approximately 10% and maintains its structure, giving stability to the battery for many cycles. Although the storage capacity of this material has allowed for the development of current portable electronic devices, it translates into a low energy density when considering its application in electric vehicles. Therefore, much of the lithium battery research is focused on the development of next generation batteries with high energy density (i.e. lighter batteries). However, enabling stable cycling and high-power output is also desirable for next generation batteries. Operation of lithium batteries at high power often leads to a decrease in the cycle life. Often a high-power output is required for a short duration, for example for rapid vehicle acceleration. The developed electrode material is cheap and easy to make, and is applicable to these high power applications.
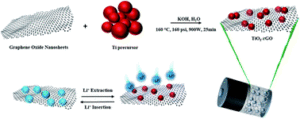
The article “A stable TiO2–graphene nanocomposite anode with high rate capability for lithium-ion batteries” focuses on the development of new electrode materials for lithium batteries that offers stable cycle performance at high power density. Titanium dioxide is an abundant metal oxide that is widely used as a pigment in paint, sunscreen, and food coloring. Titanium dioxide can be used as a stable negative electrode material, enabling long life for the battery. However, it has low electrical conductivity, thus combining the titanium dioxide with a conducting additive has been found to enhance its performance. In this study, the high power stable cycling performance of a titanium dioxide/graphene composite, prepared by a simple synthesis method, was demonstrated. The new electrode material could be used in the next generation batteries, which will be much safer and with higher power density and longer cycle life than ever before. It should be noted that this work was performed with the collaboration between battery research groups at the Helmholtz Institute of Ulm, the University of Waterloo, and the University of Calgary.
I thank Dr. Parasuraman Selvam and Dr. Edward P. L. Roberts for their cordial responses.
Read the articles:
Electrochemical performance of nano-sized LiFePO4-embedded 3D-cubic ordered mesoporous carbon and nitrogenous carbon composites
Sourav Khan, Rayappan Pavul Raj, Talla Venkata Rama Mohan and Parasuraman Selvam
RSC Adv., 2020, 10, 30406–30414
A stable TiO2–graphene nanocomposite anode with high rate capability for lithium-ion batteries
Umer Farooq, Faheem Ahmed, Syed Atif Pervez, Sarish Rehman, Michael A. Pope, Maximilian Fichtner and Edward P. L. Roberts
RSC Adv., 2020,10, 29975-29982
About the Web Writer:
 Cristian M. O. Lépori is Doctor in Chemical Sciences and currently has a postdoctoral position at the “Enrique Gaviola” Institute of Physics, CONICET, National University of Córdoba (Argentina). He works in the area of nuclear magnetic resonance studying hybrid materials formed with porous matrices and ionic liquids for use in lithium batteries. He likes to plan, organize and carry out science dissemination activities. You can find him on Twitter at @cristianlepo.
Cristian M. O. Lépori is Doctor in Chemical Sciences and currently has a postdoctoral position at the “Enrique Gaviola” Institute of Physics, CONICET, National University of Córdoba (Argentina). He works in the area of nuclear magnetic resonance studying hybrid materials formed with porous matrices and ionic liquids for use in lithium batteries. He likes to plan, organize and carry out science dissemination activities. You can find him on Twitter at @cristianlepo.
 Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.