Zhang et al. demonstrate a simple methodology for the synthesis of mechanically tough and self-healing materials.
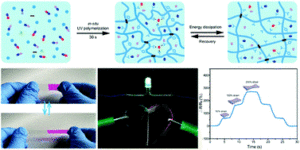
Polymers that simultaneously exhibit high mechanical toughness and self-healing properties are essential to develop the next generation of materials for various applications including optoelectronics, sensors and healthcare devices. In this work, He, Li and co-workers developed a new route for the synthesis of a tough and self-healing supramolecular network by introducing Al(III)-carboxyl complexes into photo-polymerizable deep eutectic solvent. The resulting elastomers were mechanically tough and self-healing and demonstrated high transparency and stretchability as well as ionic conductivity. In particular, thanks to the synergic interaction of H-bonds and coordination bonds within the polymer matrix, over 80% self-healing efficiency for the damaged film could be obtained after 72 hours at ambient temperature. At the same time, the interaction between the fractures interfaces allows for the quick recovery of ion channels, therefore inducing electrical self-healing properties. The materials have been thoroughly characterized by a number of techniques including nuclear magnetic resonance, electrochemical impedance spectroscopy, differential scanning calorimetry, dynamic mechanical analysis, and tensile experiments. The developed methodology is simple, environmentally friendly and fast. The authors envision that their fundamental design concept will significantly contribute to the development of the next generation of tough and self-healing materials for potential use in flexible electronics and other applications.
Tips/comments directly from the authors:
- Although in this paper we only report on the preparation of transparent conductive polymers from Al(III)-based polymerizable deep eutectic solvents (PDESs), this method can also be extended to other types of metal cations such as copper, zinc, cobalt and nickel ions. However, considering that some metal ions are coloured, the prepared conductive elastomers may be suitable for other areas of application.
- During the experiments, we also found that PDES with trivalent iron ions could not be polymerized under UV light. The reasons for this phenomenon are not yet clear and we will continue to investigate.
- The prepared transparent conductive elastomers are somewhat hygroscopic and therefore need to be kept dry during storage and use to maintain their performance stability.
- If a high transparency film is desired, a release film should be necessary to insulate the polymer from oxygen during polymerization to maintain the homogeneity of the polymer structure.
Citation to the paper: Mechanically tough yet self-healing transparent conductive elastomers obtained using a synergic dual cross-linking strategy, Polym. Chem., 2021,12, 2016-2023, DOI:10.1039/D0PY01760D
Link to the paper:
https://pubs.rsc.org/en/content/articlepdf/2021/py/d0py01760d
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.