Zhao et al. accelerate antimicrobial polymer design with a novel database and machine learning.
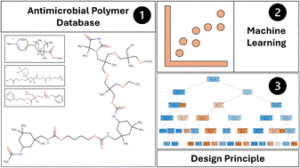
Drug-resistant microorganisms pose increasing pressure on healthcare systems, underscoring the urgent need for effective disinfectants. In this vein, antimicrobial polymers (AMPs) offer a promising solution by inhibiting or eradicating microbes through diverse mechanisms without fostering resistance. With low toxicity and enhanced durability, AMPs are strong candidates for next-generation antimicrobial agents. However, the design of new AMPs with desired properties is challenging as it is mostly based on time and labor-intensive trial-and-error research.
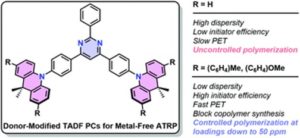
To address this, Le and collaborators gathered experimental AMP data from various sources, providing a crucial foundation for accelerating AMP design through machine learning (ML). This first open-source database for antimicrobial polymers features 489 entries, including 177 unique polymers that exhibit a range of structures and properties. Advanced algorithms were implemented to identify 32 key descriptors using seven distinct feature selection methods. Additionally, several machine learning models that achieved approximately 85% predictive accuracy for antimicrobial properties were developed. The significance and influence of these descriptors on the antimicrobial characteristics of polymers and proposed guidelines for designing highly effective AMPs were thoroughly assessed.
In summary, this study provides a database, along with the identification of critical descriptors that are expected to offer a strong and informative foundation for researchers to investigate new AMPs in the future.
Tips/comments directly from the authors:
- Data quality is crucial: Accurate, well-prepared data is foundational to the success of machine learning models and the usefulness of new knowledge extracted from such models.
- Balance novelty with rigor: Innovative methods are highlighted and ensure the approach and conclusions are rigorous and well-validated.
- Iterate and take feedback seriously: Multiple revisions help improve clarity. Feedback from peers and reviewers must be taken seriously and addressed appropriately.
Citation of the paper: Advancing antimicrobial polymer development: a novel database and accelerated design via machine learning, Polym. Chem., 2024, 15, 4063-4076.
Link to the paper: https://pubs.rsc.org/en/content/articlelanding/2024/py/d4py00736k
Link to authors website (or social media)
https://au.linkedin.com/in/tu-le-4b758a5a
https://www.rmit.edu.au/contact/staff-contacts/academic-staff/l/le-dr-tu

Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology at the University of Crete in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers. You can follow Kelly on twitter @KellyVelonia.