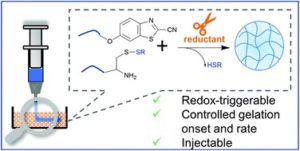
Jin et al. employ redox activated macromers to achieve precise control over the gelation onset and kinetics of a redox-triggerable, firefly luciferin-inspired hydrogel platform.
Stimuli-responsive hydrogels are porous crosslinked networks which have recently attracted tremendous interest thanks to their unique response to external stimuli allowing to tune their properties on demand. In particular, chemically-responsive hydrogels that can be actuated via mild redox reactions are quite promising for biomedical applications once biocompatible and non-cytotoxic. Despite the remarkable progress in the field, lack of precise triggering of the gelation onset and control over the rate of the gelation process of responsive hydrogels restricts a range of applications. In their current contribution, Paez and coworkers report on a novel firefly luciferin-inspired hydrogel platform endowed with redox-triggering ability and tunability of its mechanical and biological properties. To achieve this goal, protected macromers (PEG-Cys(SR)) which can be activated in the presence of a mild reductant were used as hydrogel polymeric precursors. This design allowed to in situ trigger gel formation and achieve a high degree of control. Importantly, the gelation onset and rate could be fine-tuned via altering the molecular characteristics of the precursors (e.g., structure of the protecting group, reductant type) and/or the environmental parameters of the deprotection reaction (e.g., pH, temperature). Specifically, gelation could be achieved in times spanning from seconds (CBT–Cys(SEt) / TCEP) to hours (CBT–Cys(StBu)/DTT) using precursors with good long-term stability upon storage in physiologically-relevant aqueous conditions. Furthermore, high stem cell viability was observed after 1–3 days of encapsulation in biofunctionalized CBT–Cys(SR) hydrogels. The authors anticipate that the precise control over the gelation onset and kinetics of this this redox-triggerable system, will facilitate its use for drug delivery and tissue engineering as well as inks for extrusion-based printing of soft constructs for regenerative medicine.
Tips/comments directly from the authors:
- We engineered macromers at the molecular level to achieve highly controlled gelation onset and kinetics. In the rheological characterization, it is recommended to prepare the hydrogel formulation by mixing polymer precursors solution and reductant solution in equal volume. This enables better mixing of the components and ensures good reproducibility of the rheological measurements. Two polymer precursor solutions can be mixed in advance (first half of the total volume) before adding the reductant (second half of the total volume) and loading to the rheometer.
- We evaluated the cytocompatibility of the redox-triggerable hydrogels and found excellent cell viability after 1-3 days culture. Before doing 3D cell encapsulation, it is recommended to perform a preliminary experiment of gel preparation under same conditions but in the absence of cell suspension to estimate the gelation time. Cell suspension can be replaced by cell culture medium.
Citation to the paper: Redox-triggerable firefly luciferin-bioinspired hydrogels as injectable and cell-encapsulating matrices, Polym. Chem., 2022, 13, 5116-5126, DOI: 10.1039/D2PY00481J
Link to the paper:
https://pubs.rsc.org/en/content/articlehtml/2022/py/d2py00481j
The Paez laboratory at the University of Twente:
Twitter account: @PaezLab
ResearchGate account: https://www.researchgate.net/profile/Julieta-Paez
 |
Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology at the University of Crete in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymersYou can follow Kelly on twitter @KellyVelonia
|