The 5th International ChemComm Symposium got off to an excellent start in Kyoto, Japan, on Monday (16th May) under the Chairmanship of ChemComm Editorial Board member and distinguished Professor Keiji Maruoka (Kyoto University).
World leading authorities from the USA, UK, the Netherlands, China and Japan arrived on Sunday in time to be treated to a ten course western-style dinner. The dinner was a perfect start to proceedings but even the ten courses could not over-shadow the science that was to follow.
 With an audience in excess of 100, I opened the symposium and thanked the local organisers, speakers and poster presenters. Special thanks were also given to Professor Maruoka for all his support in organising the event. As the first session Chair, Professor Maruoka then got things underway.
With an audience in excess of 100, I opened the symposium and thanked the local organisers, speakers and poster presenters. Special thanks were also given to Professor Maruoka for all his support in organising the event. As the first session Chair, Professor Maruoka then got things underway.
Professor Tsutomu Katsuki (Kyushu University) was up first, speaking initially about oxidation chemistry using ruthenium but then moving, like nature, to iron, for example. He was followed by Professor Kuiling Ding (Shanghai Institute of Organic Chemistry) who gave a talk of two halves, covering his group’s ongoing efforts to overcome the challenging issues in both homogenous and heterogeneous asymmetric catalysis.
A quick stop for refreshments was followed by Professor Véronique Gouverneur (University of Oxford) who spoke about her ongoing efforts to develop transition metal-catalysed reactions to generate C–F bonds. Not easy, but made even more challenging by the fact that the methods need to be incredibly quick so they can be used to incorporate hot [18F], which has a very short half-life. Such [18F] labelled compounds are used in positron emission tomography.
 Over lunch, the seven speakers interacted with the 35 poster presenters, putting them through their paces, with the five lucky winners scheduled to be announced at the end of the day.
Over lunch, the seven speakers interacted with the 35 poster presenters, putting them through their paces, with the five lucky winners scheduled to be announced at the end of the day.
The pace of the event did not slow after lunch. Professor Viresh Rawal (University of Chicago) wowed a packed auditorium with some of his latest results using H-bonded systems for asymmetric catalysis. Before the break, Professor Zhengfeng Xi (Peking University) spoke about the synthesis, unique reactivity, cooperative effect and applications of organo-di-lithio reagents.
The symposium was closed first by Professor Atsuhiro Osuka (Kyoto University), who spoke about some of his beautiful results in the area of Möbius porphrin chemistry, and then by Professor Ben Feringa (University of Groningen). Professor Feringa gave a wonderful overview of some of the ongoing research in his lab exploring chiral space in asymmetric catalysis. Some highlights included the latest examples of C–H and C–C bond formation using monodentate phosphoramidite ligands and also new results in the field of asymmetric catalysis using DNA with his colleague Professor Gerard Roelfes. The later certainly generated a number of interesting questions about the length and sequence requirements of the DNA involved in the reactions.
 After the formal poster prize presentations, the speakers and organising committee were treated to a traditional Japanese meal. Before dinner, the historic nature of the Japanese tea ceremony was explained in detail and served to the group by a Maiko (a young training Geiko). Through dinner, the speakers were also treated to some traditional Japanese singing and dancing performed by the Maiko. The music and dance showcased the four seasons of Kyoto.
After the formal poster prize presentations, the speakers and organising committee were treated to a traditional Japanese meal. Before dinner, the historic nature of the Japanese tea ceremony was explained in detail and served to the group by a Maiko (a young training Geiko). Through dinner, the speakers were also treated to some traditional Japanese singing and dancing performed by the Maiko. The music and dance showcased the four seasons of Kyoto.
The dinner finished with the speakers retiring to bed in preparation for their early flight first to Beijing and then Lanzhou, the next venue for the second of three ChemComm symposia.
Robert Eagling
Comments Off on The 5th ChemComm International Symposium gets underway….











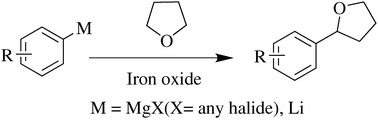
 ChemComm issue 21
ChemComm issue 21

 After the formal poster prize presentations, the speakers and organising committee were treated to a traditional Japanese meal. Before dinner, the historic nature of the Japanese tea ceremony was explained in detail and served to the group by a Maiko (a young training Geiko). Through dinner, the speakers were also treated to some traditional Japanese singing and dancing performed by the Maiko. The music and dance showcased the four seasons of Kyoto.
After the formal poster prize presentations, the speakers and organising committee were treated to a traditional Japanese meal. Before dinner, the historic nature of the Japanese tea ceremony was explained in detail and served to the group by a Maiko (a young training Geiko). Through dinner, the speakers were also treated to some traditional Japanese singing and dancing performed by the Maiko. The music and dance showcased the four seasons of Kyoto.