
Recognising significant original and independent work in supramolecular chemistry
A new probe for imaging brain tumours could offer increased hope for cancer patients, say Chinese chemists.
 Cong Li, at Fudan University, Shanghai, and colleagues have made a dendrimer-based nanoprobe called Den-Angio that can cross the blood-brain barrier. It can be used in the magnetic resonance imaging of brain tumours and should make it easier for doctors to distinguish cancerous tissue from healthy cells when cutting out the tumour.
Cong Li, at Fudan University, Shanghai, and colleagues have made a dendrimer-based nanoprobe called Den-Angio that can cross the blood-brain barrier. It can be used in the magnetic resonance imaging of brain tumours and should make it easier for doctors to distinguish cancerous tissue from healthy cells when cutting out the tumour.
To find out more, read Li’s ChemComm communication.
Luc Brunsveld and his team functionalised the two proteins with methylviologen (electron deficient) and napthalene (electron rich) guest molecules, which formed a charge transfer complex inside the CB[8] cavity. Interestingly, the resulting dimerisation can be visually observed and has established that there is distinct interplay between the supramolecular components within the proteins.
Fancy reading more? Then download the ChemComm communication,which will be free to access until the 24th June 2011.
Scientists in the UK have moved a step closer to understanding how ibuprofen could help treat cancer. The findings could lead to the drug being used as a preventative treatment for prostate cancer, in the future.
Ibuprofen – a common painkiller – can help reduce the risk of prostate cancer, but the mechanism by which it inhibits tumour cells is still not fully understood. Now, Matthew Lloyd and his team from the University of Bath in the UK, in collaboration with Cancer Research UK, have uncovered a mechanism suggesting that the chiral inversion of ibuprofen inhibits the activity of the protein alpha-methylacyl-CoA racemase (AMACR), levels of which are increased in the presence of prostate, some colon and other cancers.
To find out more, read the full news story in Chemistry World and download Lloyd’s ChemComm communication.
A highly sensitive and regenerative electrochemical immunosenor has been created by scientists in China, by combining a 3D tetrahedra-structured probe (TSP) and a DNA-bridged antibody immobilisation technique.
| Chunhai Fan and colleagues from the Shanghai Institute of Applied Physics collaborated with scientists from the Nanjing University of Science and Technology. Together the team alleviated common problems associated with electrochemical biosensors, such as poor reproducibility and orientation of surface confined molecules, by using the TSP. Next, the team assembled the TSP onto the gold surface with high reproducibility, resulting in controlled spacing and orientation of the DNA probes. Antibodies were then reversibly anchored to the nanostructured surface via a DNA bridge, producing the immunological (iTSP) platform. |
Fan believes that the iTSP platform will be a promising tool for various immunological assays, as well as DNA-programmed protein microarrays for future proteomic studies, and here’s a summary of the reasons why:-
Fancy reading more? Then download the ChemComm communication, which will be free to access until the 24th June 2011.
The international speakers assembled early on Thursday morning to make the final journey to Nankai University, Tianjin. After a flight and a two hour journey on the ChemComm minibus, we arrived at our hotel directly opposite the entrance gates to Nankai University. The first thing that struck us about the hotel was the enormous rooms. I actually got lost in mine between the two bathrooms, the study and the lounge area. Apparently they were all like this – what a hotel!
After a formal dinner with our host Professor Qilin Zhou (Director of the State Key Laboratory of Elemento-organic Chemistry and member of the Chinese Academy of Sciences), most retired to bed but Professor Rawal, Professor Maruoka and I were taken to a live Chinese comedy show. Lots of clapping with plastic clapping hands, much laughter and, of course, all sketches in Chinese. It was a great experience even if we did not understand anything apart from ‘hello’ and ‘thank you’.
The following day started with eager anticipation as an audience in excess of 100 watched Professor Feringa give his final scientific presentation on motors and switches. He was followed by local Professor of Energy, Yun Chen, who spoke about some of his latest research into new nanomaterials for batteries, a key challenge with the ever increasing energy demands around the world. Before lunch, Veronique Gouverneur gave her presentation looking at the latest developments in organofluorine chemistry.
 After lunch, the five poster judges took to their duties for one last time with each asked to examine ten posters and select just one. With the final five selected it was left to Professors Feringa and Maruoka to rank the winners. All five received money from the State Key Lab thanks to the generosity of Professor Zhou, plus journal subscriptions and books from the RSC, amongst other things, as additional gifts.
After lunch, the five poster judges took to their duties for one last time with each asked to examine ten posters and select just one. With the final five selected it was left to Professors Feringa and Maruoka to rank the winners. All five received money from the State Key Lab thanks to the generosity of Professor Zhou, plus journal subscriptions and books from the RSC, amongst other things, as additional gifts.
The scope of the meeting then switched to green chemistry as Professor Buxing Han spoke about his latest research using ionic liquids, supercritical fluids and a mixture of the two as solvents for organic reactions. Professor Han was followed by Professor Rawal who gave his third different talk in five days covering his group’s total synthesis of a member of the Welwitindolinone group of natural products. This was a particularly insightful talk as Professor Rawal not only discussed things that worked but also routes that had failed. There was certainly a good lesson here for total synthesis students, namely things may not always work out but perseverance is the key to success.
In the last session of the day, Professor Maruoka spoke about some of his latest results in the field of organocatalysis. He was followed by Zhen Yang who gave a second total synthesis masterclass. The retrosynthetic analysis slides certainly gave me a trip down memory lane and the overall synthesis in 26 steps (micrandilactone) showcased the real power of organic chemistry and the creative thought processes needed to succeed.
After the poster presentations had been made, the speakers and other key faculty members walked to a very famous restaurant called Goubuli for steamed dumplings. They are a local speciality, filled with vegetables, fish and, traditionally, pork. Normally six is enough, but I was proud to exceed the average! The other highlight of the dinner was the stinky tofu, a delicacy providing you can get past the tremendously bad smell, much stronger than the bluest blue cheese. This was a real challenge – the stink was just so bad!
After dinner, the speakers and faculty members retired to a German bar for one last drink to celebrate the day and the past week. Over the course of the seven days we had travelled many thousand of miles, seen 21 scientific talks covering many different aspects of the chemical sciences, with a total audience close to 700 and nearly 200 posters judged. All in all, it was a great week and the only thing left to do is to thank all the speakers and, of course, the local hosts, without whom none of this would have been possible.
Robert Eagling
View the Nankai Symposium schedule
Related posts:
The challenging synthesis of B-seco limonoids has been achieved using an Ireland-Claisen rearrangement, report scientists in Germany.
Herbert Waldmann worked with a collaboration of scientists from various German science institutes on this project and believes that the synthetic strategy described here might facilitate the total synthesis of other natural products containing similar limonoid scaffolds and their analogues.
For more details on the reaction conditions used, download the ChemComm communication today, which will be free to access until the 24th June 2011.
 |
You may also be interested to know that Herbert Waldmann recently helped guest edit a web themed issue on ‘Enzymes and Proteins’ – A nice collection of articles from high profile scientists in this area of chemical biology, including some of Herbert’s very own research papers. |
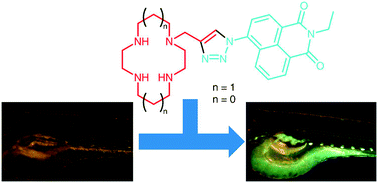
The presence of abnormally high or low level of zinc in specific organs and areas has been linked to several diseases and conditions, including Alzheimer’s disease, stroke and epilepsy. In the light of these considerations, it is not surprising that many efforts have been devoted to in vivo zinc sensing and quantification.
A significant portion of the research to uncover the correlations between the concentration of metal ions, pathologies and development is performed on animal models such as zebrafish (Danio rerio). Due to their fast development, they can be grown outside the mother’s body, and their embryos are transparent, allowing for a clear observation of their organs without the need for dissection.
Watkinson and Goldup, from Queen Mary University of London, recently focused their effort to the development of a fluorescent sensor for zinc to be used in model studies on zebrafish. The sensor is based on macrocyclic nitrogen-containing rings (cyclen or cyclam) equipped with a fluorescent pendant arm, introduced using a widely known and applied “click” cycloaddition. The sensor’s fluorescence is activated upon coordination of the zinc anion in a scorpionate fashion.
The sensors proved reliable and stable in a wide range of pH, ideal for in vivo use, and a remarkable selectivity for zinc over other possible anions. Competition experiments with twelve different anions showed that only in the presence of Fe3+ and Cu2+ in threefold excess (in relation to zinc) the sensor failed to discriminate between the metals.
To test their performance in biological models, zebrafish eggs were grown in solutions of the sensors and the distribution of fluorescence monitored during their growth, proving not toxic to the subjects. The accumulation of fluorescence concentrated in the eye, the gall bladder and the biliary system, all regions not highlighted by previous sensors, suggesting a different mechanism of absorption for these macrocyclic compounds and different cell permeability.
Although those reported are preliminary results, the characteristics of these sensors may make them a viable candidate for future applications in vivo sensing of zinc pools.
Read the article online or access the ESI (free).
Modular ‘click’ sensors for zinc and their application in vivo
Kajally Jobe, Caroline H. Brennan, Majid Motevalli, Stephen M. Goldup and Michael Watkinson
Chem. Commun., 2011, 47, 6036-6038
Posted on behalf of Dr. Giorgio De Faveri, Web Writer for Catalysis Science & Technology
This communication is part of the ChemComm Supramolecular Chemistry web themed issue. Check out the web theme page to download other contributions from this exciting issue.
Bioanalysis and biosensors represent a current area of wide interest in the chemical sciences. Much work is being put into methods that allow easy separation and detection of biological targets. Erkang Wang and coworkers at the Chinese Academy of Sciences in Changchun have presented a new way of doing just that.
The group synthesised a new type of silver micro-dendrite (SMD) that has a silver surface perfect for tethering biomolecules. The SMDs could also be reversibly separated and dispersed in water merely by oscillation and then settling under gravity for 30 seconds.
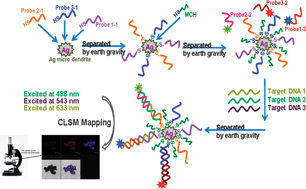
The team used the SMDs to successfully detect DNA from sickle cell disease, human T-lymphotropic virus and anthrax. Probe DNA was attached to the SMDs and then the target strands were selectively bound with a second, fluorophore-containing probe. The target DNA could then be detected using laser scanning confocal microscopy. They found that this technique was so sensitive it could even detect DNA with a one nucleotide mismatch.
Wang hopes that this type of separation system can be expanded to more targets and other types of sensing system.
Interested in finding out more? Then download the ChemComm article for free today and leave a comment below.
A 5am start was always going to be tough, particularly after such a successful event in Kyoto. That said, with cases packed, we headed to Kansai Airport…. next stop Beijing!
After a three hour flight, the ChemComm International delegation arrived at Beijing International Airport where we had a four hour layover. After lunch, we were joined by the RSC Publisher for China, Dr Daping Zhang, and Professor Keiji Maruoka, the final international speaker for the next two events. The flight to Lanzhou was smooth and, to our delight, we were met by the former director of the Key State Laboratory and member of the Chinese Academy of Sciences, Professor Yong-Qiang Tu. Following a buffet dinner accompanied by excellent regional red wine, the speakers retired early with one eye on the second symposium. 
The following morning, we were greeted at the entrance to Lanzhou University with possibly the largest ChemComm advertising sign I have ever seen. ‘Big’ was certainly the theme of the day, with Professor Wei Wang informing speakers that an audience of around 400 was anticipated with 80 posters to review over lunch.
Professor Tu opened events and chaired the first session, inviting Professor Xinhe Bao to the stage. Professor Bao gave a beautiful overview on some of his very detailed studies into heterogenous catalysis within nanotubes and other nano-confined systems. Using nobel metals or metals such as iron or nickel, it was shown that the conversation of syngas was much more efficient inside the tube than outside. The subject of the session then switched to metal-free homogenous catalysis, as Professor Maruoka spoke about some of his latest results in the field of organocatalysis. As you would expect, high enatiomeric excesses and yields were the order of the day.
Following coffee, Professor Veronique Gouverneur gave another whistle-stop tour of her group’s latest organofluorine chemistry and the formation of C–F bonds using palladium and gold homogenous catalysis.

 As in Kyoto, the speakers worked hard over lunch analysing the 80 posters on show. They covered everything from organic methodology, to total synthesis, organic materials and supramolecular chemistry. If they thought judging them was tough in Kyoto, it proved even more so in Lanzhou! Unfortunately, Professor Ben Feringa was unable to act as a judge, because, due to a last minute laptop crisis, his presentation was being transferred to a spare laptop, with his own laptop in pieces, being fixed by helpful student Mr Woo.
As in Kyoto, the speakers worked hard over lunch analysing the 80 posters on show. They covered everything from organic methodology, to total synthesis, organic materials and supramolecular chemistry. If they thought judging them was tough in Kyoto, it proved even more so in Lanzhou! Unfortunately, Professor Ben Feringa was unable to act as a judge, because, due to a last minute laptop crisis, his presentation was being transferred to a spare laptop, with his own laptop in pieces, being fixed by helpful student Mr Woo.
After lunch, the conference switched gears to total synthesis. Professor Dawei Ma spoke first about the total synthesis of galbulimima alkaloids and communesins. Professor Ma was followed by Professor Viresh Rawal, who gave a different talk to that described in the programme. Professor Rawal described his recent total synthesis of Pederin and Mycalamide B, both part of the Pederin family of natural products isolated from the beetle Paederus fuscipas. He also introduced initial work looking at bioactivity of Mycalamide in cells, carried out with Milan Mrksich.
 For the final session, the audience were treated to a masterclass from Ben Feringa on molecular motors and switches. This was only made possible by Mr Woo, who had rebuilt his computer, replacing a transistor, and bingo! His synthetic motors, inspired by nature, are unidirectional with the direction controlled by the enantiomer used. By adding legs, the motors can be bound to gold surfaces and then used to change the orientation of liquid crystal films. A great talk and even Professor Bao, the session Chair, could not bring himself to cut it short. Thank goodness for Mr Woo! The final talk of the day was presented by Professor Deqing Zhang on tetrathiafulvalene-based switchable molecular systems towards functional materials.
For the final session, the audience were treated to a masterclass from Ben Feringa on molecular motors and switches. This was only made possible by Mr Woo, who had rebuilt his computer, replacing a transistor, and bingo! His synthetic motors, inspired by nature, are unidirectional with the direction controlled by the enantiomer used. By adding legs, the motors can be bound to gold surfaces and then used to change the orientation of liquid crystal films. A great talk and even Professor Bao, the session Chair, could not bring himself to cut it short. Thank goodness for Mr Woo! The final talk of the day was presented by Professor Deqing Zhang on tetrathiafulvalene-based switchable molecular systems towards functional materials.
The evening social events were as memorable as the science, with the speakers joined by 50 or so guests including the vice Dean of Lanzhou University. The Chinese banquet was enhanced with locally produced red wine and Chinese liquor (only to be drunk in multiples of three – Chinese style…kambai). After many toasts and speeches, the speakers were taken to a local pub for some traditional Chinese music. The night was not done, however, as we then moved to a Tibetan bar. The speakers were told to expect local singing and dancing. Little did they know that they would be involved! After being sung to, each speaker was presented with a white Hada. What a great way to end such a action-packed day.
Next stop Tianjin for the final event of three…..
Robert Eagling