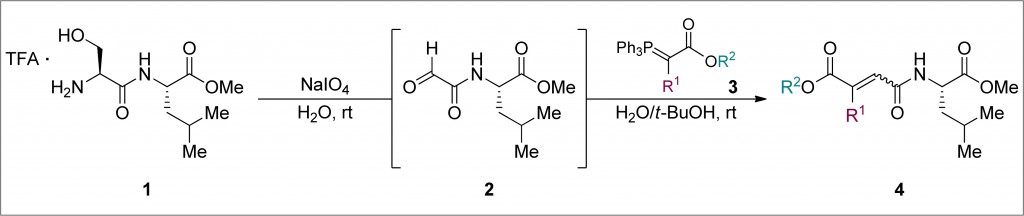
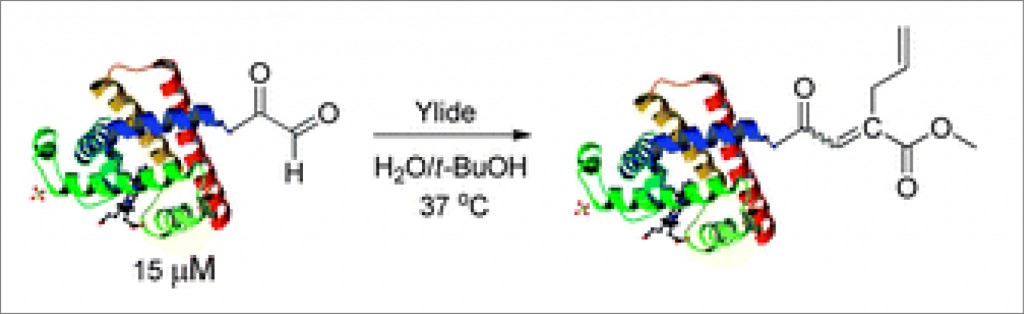
Researchers in Italy have shown that medicinal gold compounds interact strongly with the proteins of the copper trafficking system, which could have implications for drug delivery.
The copper trafficking system consists of proteins that help the uptake of copper into cells and then promote its transfer and delivery to copper-dependent cellular proteins. One of these ‘chaperones’ is known as Atox-1.
Previous work has shown that platinum-based anticancer drugs strongly interact with copper trafficking system proteins and Messori and co-workers hypothesised that medicinal gold compounds might also do the same, especially in the +1 oxidation state; soft lewis acids, such as gold (I) ions react eagerly with Atox-1.
The interactions of three gold (III) compounds with Atox-1 were analysed through ESI-MS and revealed the formation of metal-protein adducts. The same major adduct was invariantly formed, matching the protein binding of a single gold (I) ion. Formation of this adduct implied that the gold (III) complex had broken down, a loss of ligands and reduction to a gold (I) species. ESI-MS also displayed peaks that corresponded to protein binding with two gold (I) ions. A stability study showed that one of the three gold-protein adducts was stable over 72 hours.
From their findings, the authors conclude that the cytotoxic gold compounds investigated form stable adducts with copper chaperone, Atox-1. These results have implications for medicinal drug design and our little friend, Atox-1 stays in a job.
Read this HOT Chem Comm article today (free to access until the 14th of December 2012):
Medicinal gold compounds form tight adducts with the copper chaperone Atox-1: biological and pharmacological implications
Chiara Gabbiani, Federica Scaletti, Lara Massai, Elena Michelucci, Maria A. Cinellu and Luigi Messori
Chem. Commun., 2012, 48, 11623-11625
Published on behalf of Sarah Brown, Chemical Communications web science writer.











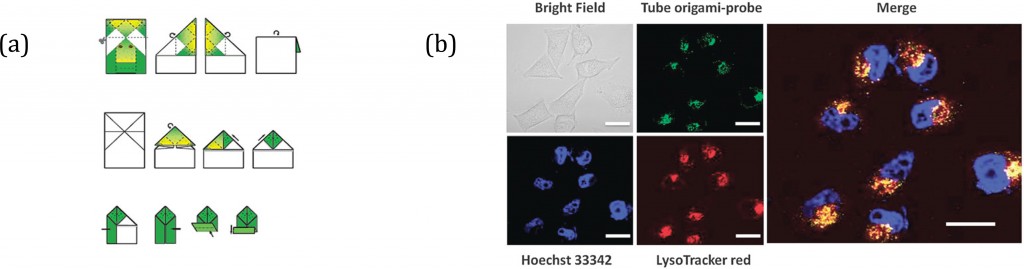
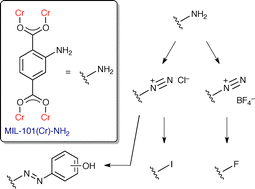 Burrows et al.
Burrows et al.