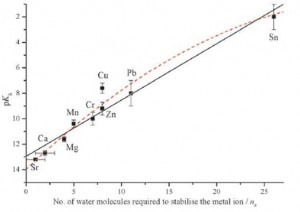
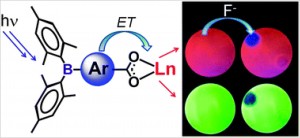
 Luminescence from lanthanide-based compounds is currently exploited in electroluminescent devices ranging from glucose monitoring sensors to thin-film displays. Triarylboron groups are known to greatly enhance the fluorescence or phosphorescence of transition metal complexes, but such compounds are very sensitive to oxygen quenching and are unsuitable for use as sensors under ambient conditions. The combination of these groups with lanthanides may offer enhanced luminescence in more ambient-friendly lanthanide-based materials.
Luminescence from lanthanide-based compounds is currently exploited in electroluminescent devices ranging from glucose monitoring sensors to thin-film displays. Triarylboron groups are known to greatly enhance the fluorescence or phosphorescence of transition metal complexes, but such compounds are very sensitive to oxygen quenching and are unsuitable for use as sensors under ambient conditions. The combination of these groups with lanthanides may offer enhanced luminescence in more ambient-friendly lanthanide-based materials.
The Wang group from Queen’s University, Canada have synthesised the first examples of triarylboron functionalized Tb(III) and Eu(III) compounds, and have shown that the BMes2 group is extremely effective at activating lanthanide emissions. The new compounds have been tested as sensors, showing that such triarylboron functionalized lanthanides may be promising new CN– and F– anion sensors.
To find out more, download this HOT article now (free to access until the 5th of December 2012).
Maria Varlan, Barry A. Blight and Suning Wang
Posted on behalf of Katie Renouf, Chemical Communications web science writer.











 Lowering the drug dose required to effectively treat a patient would save money and reduce side effects. To achieve this, one area of research is focusing on using a ‘prodrug’ approach in which less toxic substrates, or prodrugs, are given to a patient and are converted to the active drug form by an enzyme only at a specific site.
Lowering the drug dose required to effectively treat a patient would save money and reduce side effects. To achieve this, one area of research is focusing on using a ‘prodrug’ approach in which less toxic substrates, or prodrugs, are given to a patient and are converted to the active drug form by an enzyme only at a specific site.

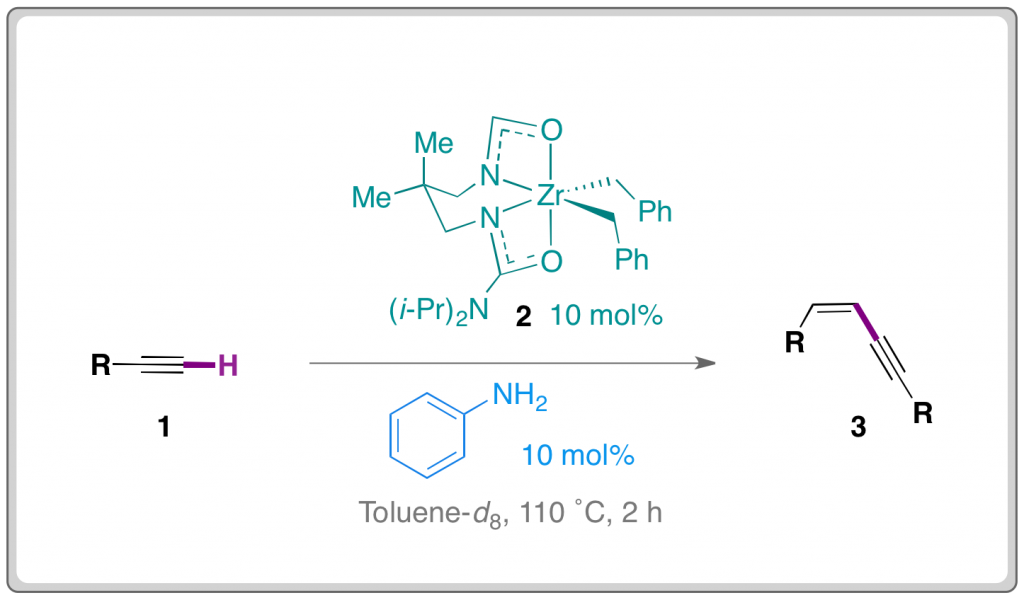

 Costly metals in some solar cells could be replaced by cheap resins, according to Korean research.
Costly metals in some solar cells could be replaced by cheap resins, according to Korean research.