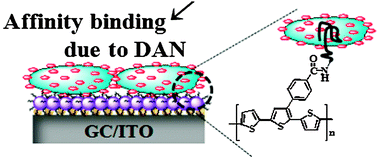
Alzheimer’s disease is the most common form of dementia and, as there is no cure, early diagnosis is crucial for treatment to be effective. To this end, UK and US scientists have developed a labelled tracer compound that binds to plaques closely associated with Alzheimer’s disease (AD) so that the plaques can be picked up by a medical imaging technique.
The tracer compound is a [18F]-labelled barbiturate and is used with the imaging technique positron emission tomography (PET). Although other radiolabelled compounds have been used as PET tracers, using [18F]-labelled barbiturates for molecular imaging in AD has distinct advantages, such as good blood-brain barrier crossing ability, metabolic stability and easy accessibility.
 As Alzheimer’s disease advances, symptoms can include confusion, irritability and aggression, and long-term memory loss © Shutterstock
As Alzheimer’s disease advances, symptoms can include confusion, irritability and aggression, and long-term memory loss © Shutterstock
Matteo Zanda at the University of Aberdeen and colleagues, in conjunction with Pfizer in the US, developed several fluorinated barbiturate analogues. The key to developing an effective molecular imaging radiotracer is the ability to distinguish between a healthy individual and someone suffering from a neurological disease, such as AD, they say. Barbiturates have a strong capacity for forming structures with biopolymers and are effective metal ion chelators. As such, the team thought that they would bind to AD-related plaques, which consist of the biopolymer β-amyloid and metal cations, such as Zn(II) and Cu(II).
See the Chemistry World story in full or read the Chem Comm article:
18 F-barbiturates are PET tracers with diagnostic potential in Alzheimer’s disease
Elisa Calamai , Sergio Dall’Angelo , David Koss , Juozas Domarkas , Timothy J. McCarthy , Marco Mingarelli , Gernot Riedel , Lutz F. Schweiger , Andy Welch , Bettina Platt and Matteo Zanda
Chem. Commun., 2013,49, 792-794
DOI: 10.1039/C2CC38443D
Comments Off on Early Alzheimer’s diagnosis compound











 As Alzheimer’s disease advances, symptoms can include confusion, irritability and aggression, and long-term memory loss © Shutterstock
As Alzheimer’s disease advances, symptoms can include confusion, irritability and aggression, and long-term memory loss © Shutterstock


![spinksbanner2_tcm18-223978[1]](https://blogs.rsc.org/md/files/2012/11/spinksbanner2_tcm18-2239781.jpg)