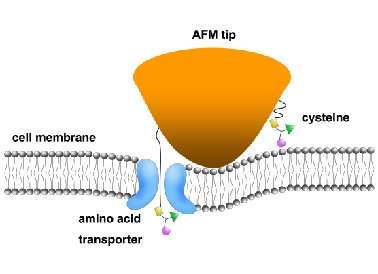
A gel that can move backwards and forwards in a tube in response to changes in light intensity has been developed by an international team of chemists. As it can change its direction of travel, the gel could one day be used as a material to allow tiny intelligent robots to approach more favourable environments or flee advere stimuli.
The gel’s ability to move is thanks to the Belousov–Zhabotinsky (BZ) reaction. BZ reactions are known for their unusual oscillatory properties caused by the non-equilibrium thermodynamics of the reaction. By including the BZ reaction in specific gels, it is possible to obtain soft materials which can show repeated swelling and shrinking on a scale that can even push objects through a tube.
When one end of a BZ gel is made to oscillate faster than the other…

Gel moving away from the light (left) and gel moving towards the light (right)
Read the full article in Chemistry World
Read the original journal article in ChemComm:
Photophobic and phototropic movement of a self-oscillating gel
Xingjie Lu, Lin Ren, Qingyu Gao, Yuemin Zhao, Shaorong Wang, Jiaping Yang and Irving R. Epstein
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC44480E, Communication