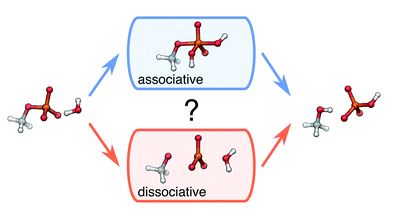
A group of researchers based in Valencia, Spain, have developed an enantio- and diastereoselective method to synthesize oxazolines, using a combination of silver and an organocatalyst that is only one degree of separation away from a classic British cocktail (to get that one, you might have to keep reading). This method complements the current literature by employing unactivated ketone substrates to prepare products with two adjacent chiral centres, one of which is quaternary.
Oxazolines are a versatile functional group, found in synthetic precursors and functional molecules and materials. They are a common structural feature in many natural products and in medicinal chemistry they are incorporated into synthetic drug candidates as bioisosteres of other functional groups, such as thiazoles and imidazoles. Oxazolines can also be used to prepare poly(2-alkyl/aryl-oxazoline) polymers, which have utility in biomedical fields to prepare hydrogels, nanoparticles for drug delivery and imaging, and polymer-protein conjugates. In an organic synthesis laboratory oxazolines are encountered as ligands in asymmetric catalysis, such as the bis(oxazoline) ‘BOX’ family of compounds.
![Synthesis of oxazolines from ketones and isocyanaoacetate esters via a formal [3+2] cycloaddition reaction catalysed by a multicatalytic system of silver and a dihydroquinine squaramide organocatalyst](https://blogs.rsc.org/cc/files/2018/04/Screenshot-2018-04-03-16.24.41-300x171.png)
Synthesis of oxazolines from ketones and isocyanaoacetate esters via a formal [3+2] cycloaddition reaction.
The researchers optimised the reaction with acetophenone and
tert-butyl isocyanoacetate, and found that Ag
2O (2.5 mol%) and a dihydroquinine squaramide organocatalyst (5 mol%) promoted the reaction to obtain a quantitative yield of the
cis-oxazoline (80:20
cis/trans) in 24 hours with excellent enantiomeric excess (99/93% e.e.). The researchers tested the reaction scope and found that acetophenone derivatives substituted with electron-withdrawing and donating groups (NO
2, Cl, Br, Me) gave the
cis-oxazoline products in reliable yields (60 – 99%), fair to good diastereoselectivity (56:44 – 95:5
cis/
trans) and excellent enantioselectivities (91 – 99% e.e.). Other examples tested included cyclohexanone, 2-acetylthiophene, deoxybenzoin and acetone as well as aliphatic ketones with a combination of methyl, isopropyl and cyclopropyl groups (63-99% yield, 45:55 – 98:2
cis/
trans, 56 – 98% e.e.).
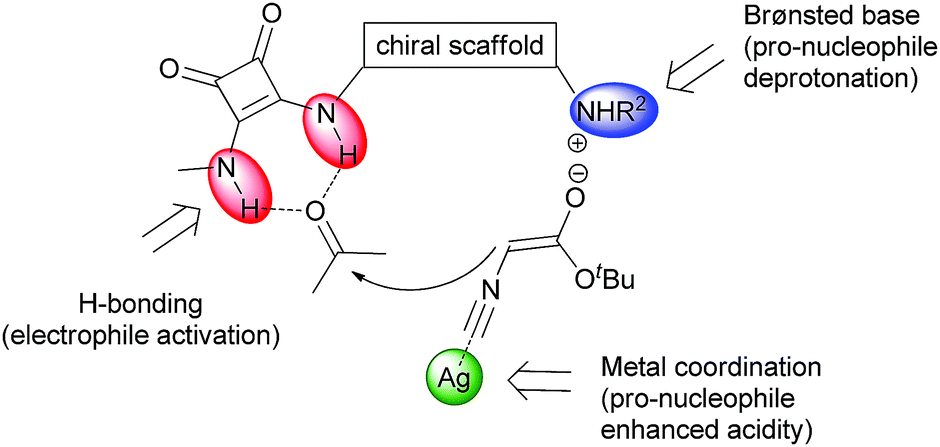
Diagram showing the triple activation of substrates using a dihydroquinine squaramide organocatalyst combined with a silver lewis acid.
The dihydroquinine squaramide organocatalyst used is a composite structure comprising a rigid cyclobutendione ‘squaramide’ and a reduced quinine analogue. The former makes strong hydrogen bonds with carbonyl derivatives while the latter confers chirality on the catalyst and has a Brønsted basic quinuclidine moiety. Quinine is an alkaloid isolated from the cinchona plant, and is the same stuff in anti-malarial tonic that is better washed down with a measure of gin. The reaction proceeds via silver-coordination to the terminal carbon of the isocyanate, accelerating deprotonation of the alpha protons by the quinuclidine base and forming a nucleophilic enoate. The electrophilicity of the ketone is enhanced by the formation of two hydrogen bonds with the N-H bonds of the squaramide, shaping a doubly activated system which rapidly undergoes a formal [3+2] cycloaddition to give the products.
At first glance this seems to be a simple and straightforward transformation, but in reality the authors have succeeded in optimising a reaction for unactivated substrates which has stereocontrol over two chiral centres, one of which is quaternary. Furthermore, the reaction uses a combination of two catalysts reacting via three modes of activation.
To find out more please read:
Enantioselective synthesis of chiral oxazolines from unactivated ketones and isocyanoacetate esters by synergistic silver/organocatalysis
Pablo Martínez-Pardo, Gonzalo Blay, M. Carmen Muñoz, José R. Pedro, Amparo Sanz-Marco and Carlos Vila.
Chem. Commun., 2018, 54, 2862 – 2865
DOI: 10.1039/c8cc00856f
 About the author:
About the author:
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.
Comments Off on Synthesis of Oxazolines with a Triple Activation Twist
 ___________________________________________
___________________________________________ ___________________________________________
___________________________________________ ___________________________________________
___________________________________________ ___________________________________________
___________________________________________ ___________________________________________
___________________________________________










![Synthesis of oxazolines from ketones and isocyanaoacetate esters via a formal [3+2] cycloaddition reaction catalysed by a multicatalytic system of silver and a dihydroquinine squaramide organocatalyst](https://blogs.rsc.org/cc/files/2018/04/Screenshot-2018-04-03-16.24.41-300x171.png)


 Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at 
 Dr. Rafal Klajn
Dr. Rafal Klajn