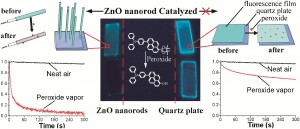
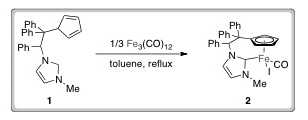
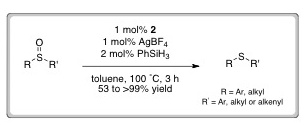
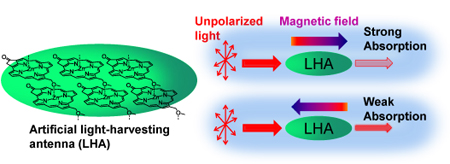
LAST CALL FOR ORAL ABSTRACTS – DEADLINE THIS FRIDAY 11TH MAY 2012
Directing Biosynthesis III (DBIII), 19 – 21 September 2012, University of Nottingham, UK
Please don’t miss this last opportunity to submit an oral abstract now to be part of a high profile conference featuring contributions from the most active groups in the UK, Europe, the USA and Japan working in this rapidly developing area.
This meeting builds on the two previous extremely successful conferences in a subject area which remains highly topical. As significant opportunities exist for engineering biosynthetic pathways in bacteria, fungi and plants for the directed biosynthesis of new natural products with new and beneficial properties. We expect the programme this year to generate a high profile event that you will not want to miss.
Confirmed Invited speakers:
Ikuro Abe, University of Tokyo, Japan
Mervyn Bibb , John Innes Centre, UK
David W. Christianson , University of Pennsylvania, USA
Christian Hertweck , Friedrich Schiller University Jena, Germany
Ben Liu , The University of Texas at Austin, USA
Professor Jim Naismith , University of St Andrews, UK
Joern Piel , University of Bonn, Germany
Professor Chris Schofield , University of Oxford, UK
David H Sherman , University of Michigan, USA
Dr David R Spring , University of Cambridge, UK
Tom Simpson , FRS, University of Bristol, UK
Yi Tang , UCLA, USA
NOW CONFIRMED – Craig Townsend , John Hopkins University, USA
A special symposium will take place within the Directing Biosynthesis III programme, recognising the achievements of three 2011 RSC award winners. Each of the winners will give a keynote lecture within the symposium.