UK scientists have shown that the sidewalls and closed ends of carbon nanotubes can support fast electron transfer, challenging the belief that they are electrochemically inert.
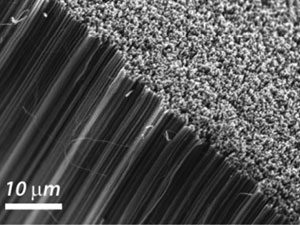
Carbon nanotubes (CNTs) have wide ranging electrochemical applications for sensing and energy. Forests of vertically aligned CNTs have been proposed for use as electrodes, but it was thought that the inert sidewalls would have to be insulated and the ends opened to allow electron transfer.
Scientists from the University of Warwick have now challenged this position by showing that the sidewalls and closed ends of CNTs can support fast electron transfer.
See the full article in Chemistry World
Link to journal article
Electrochemistry at carbon nanotube forests: sidewalls and closed ends allow fast electron transfer
Thomas S. Miller, Neil Ebejer, Aleix G. Güell, Julie V. Macpherson and Patrick R. Unwin
Chem. Commun., 2012, Advance Article, DOI: 10.1039/C2CC32890A, Communication












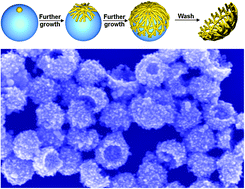 A gold nanocup – it sounds like something a posh fairy might drink out of. But actually, metal nanocups are promising particles for sensing and nanoelectronics thanks to their plasmon coupling and light scattering properties. Until now, they have been difficult to make but
A gold nanocup – it sounds like something a posh fairy might drink out of. But actually, metal nanocups are promising particles for sensing and nanoelectronics thanks to their plasmon coupling and light scattering properties. Until now, they have been difficult to make but 
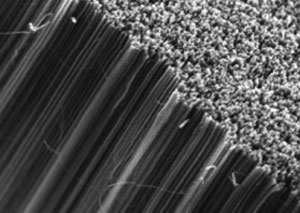 3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?
3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?