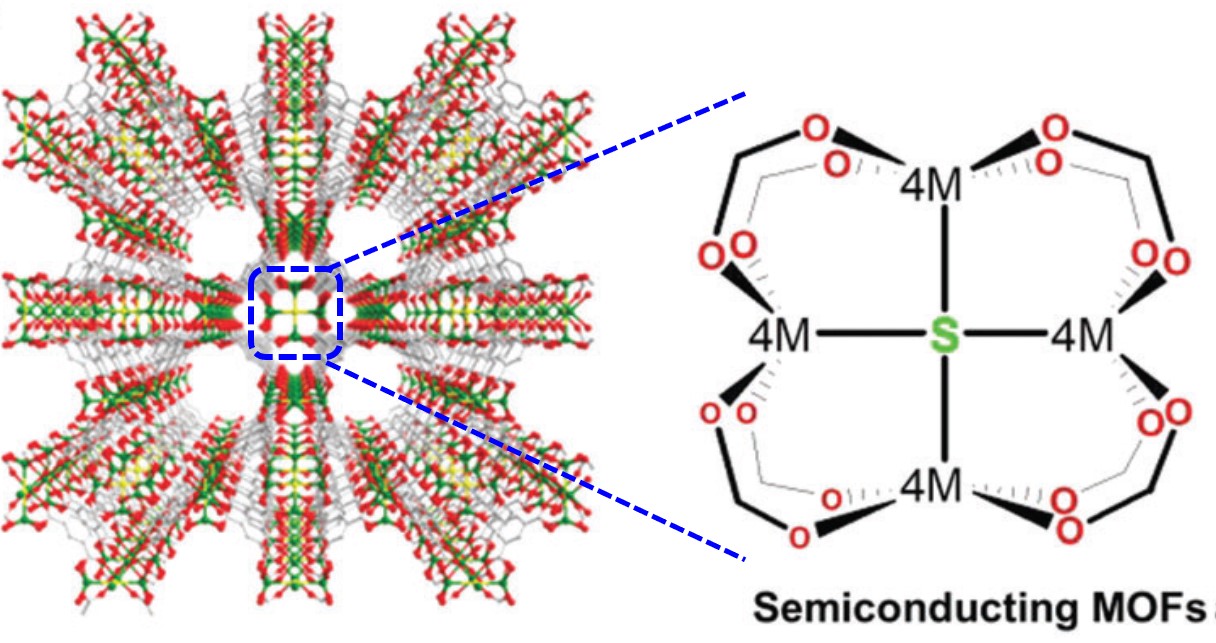
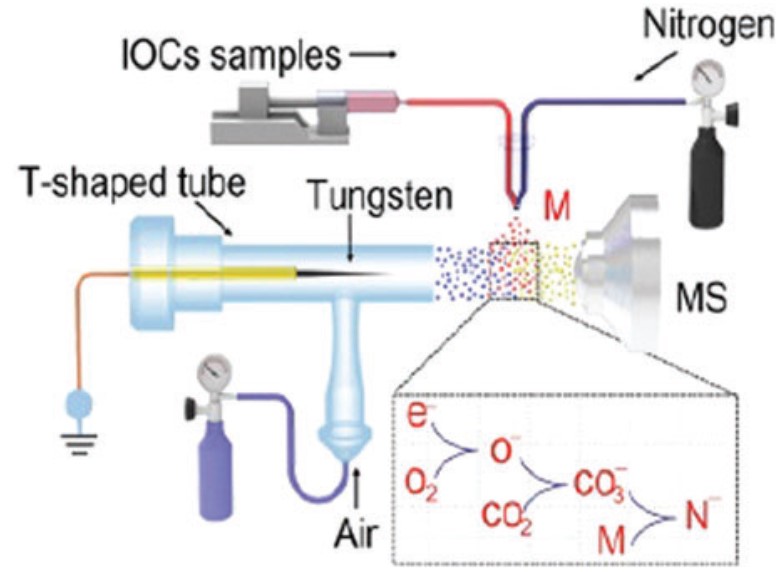
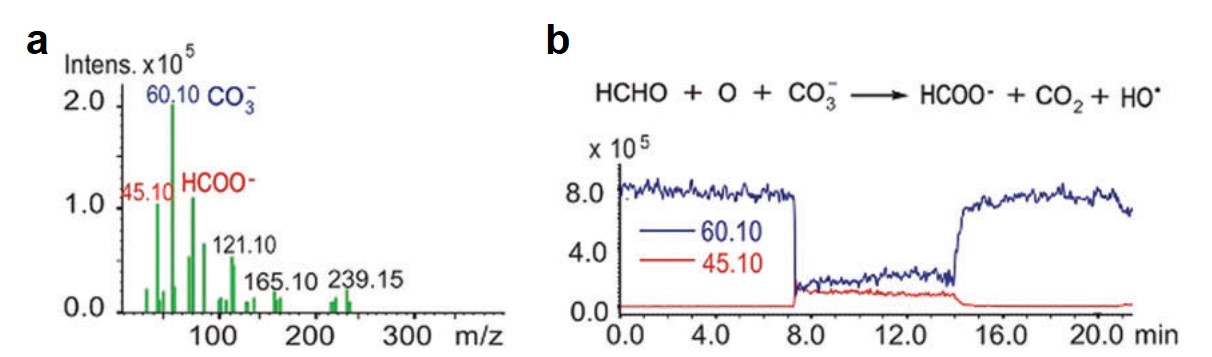
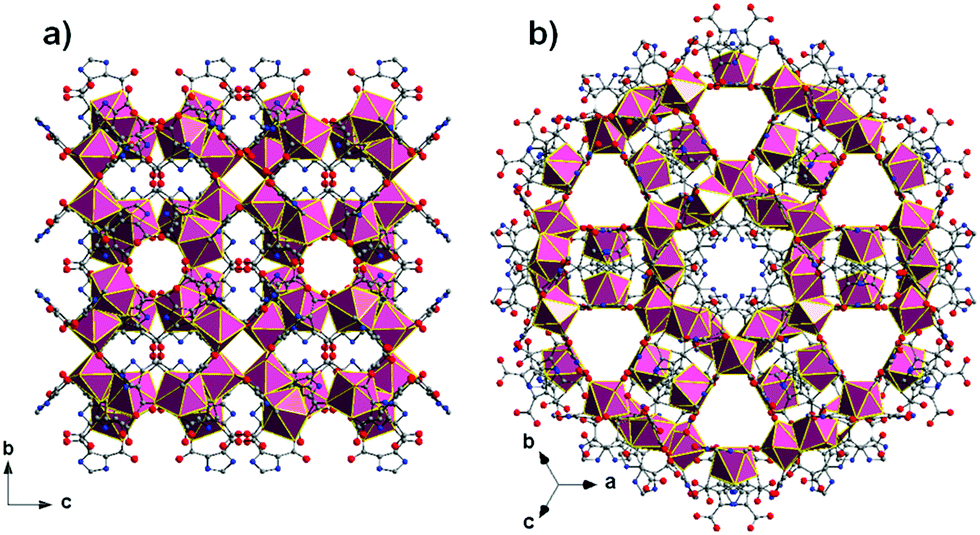
All of the referee-recommended articles below are free to access until Monday 1st April.
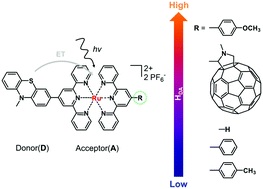
Remote control of electronic coupling – modification of excited-state electron-transfer rates in Ru(tpy)2-based donor–acceptor systems by remote ligand design
Yusen Luo, Jens H. Tran, Maria Wächtler, Martin Schulz, Kevin Barthelmes, Andreas Winter, Sven Rau, Ulrich S. Schubert and Benjamin Dietzek*
Chem. Commun., 2019, 55, 2273-2276
DOI: 10.1039/C8CC10075F, Communication
___________________________________________________________
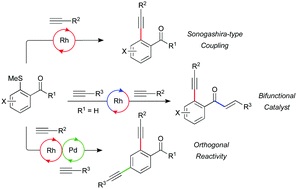
A rhodium-catalysed Sonogashira-type coupling exploiting C–S functionalisation: orthogonality with palladium-catalysed variants
Milan Arambasic, Manjeet K. Majhail, Robert N. Straker, James D. Neuhaus and Michael C. Willis*
Chem. Commun., 2019, 55, 2757-2760
DOI: 10.1039/C9CC00092E, Communication
___________________________________________________________
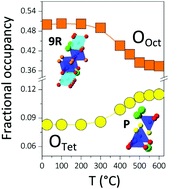
Hexagonal perovskite derivatives: a new direction in the design of oxide ion conducting materials
Sacha Fop,* Kirstie S. McCombie, Eve J. Wildman, Janet M. S. Skakle and Abbie C. Mclaughlin*
Chem. Commun., 2019, 55, 2127-2137
DOI: 10.1039/C8CC09534E, Feature Article
___________________________________________________________
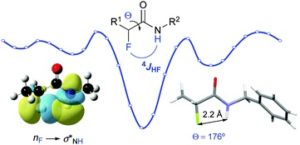
Combined experimental and theoretical study of long-range H–F interactions in α-fluoro amides
Elena Cosimi, Nils Trapp, Marc-Olivier Ebert* and Helma Wennemers*
Chem. Commun., 2019, 55, 2253-2256
DOI: 10.1039/C8CC09987A, Communication
___________________________________________________________
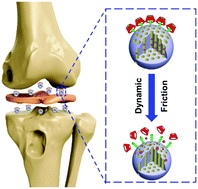
Mechanised lubricating silica nanoparticles for on-command cargo release on simulated surfaces of joint cavities
Xiaolong Tan, Yulong Sun, Tao Sun and Hongyu Zhang*
Chem. Commun., 2019, 55, 2593-2596
DOI: 10.1039/C8CC10069A, Communication
___________________________________________________________
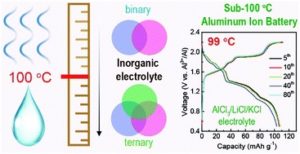
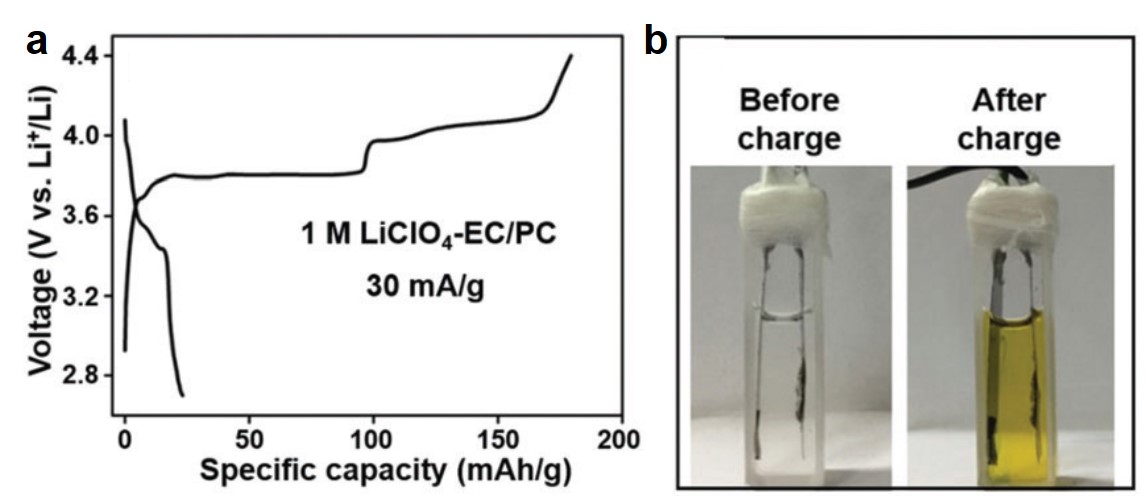
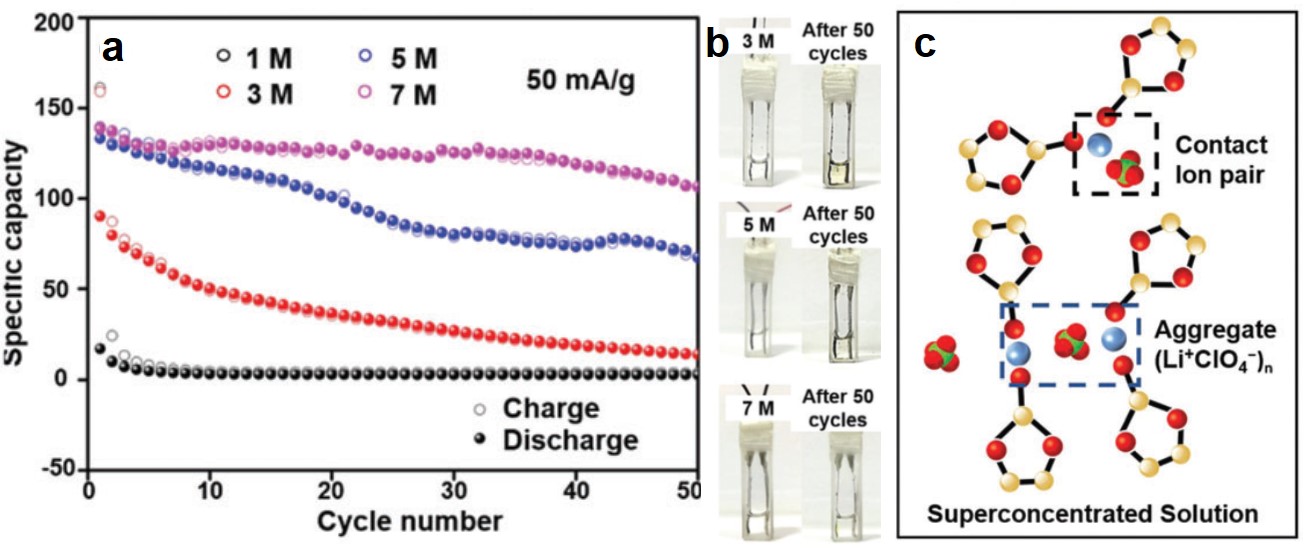
A sub-100 °C aluminum ion battery based on a ternary inorganic molten salt
Jie Wang, Xu Zhang, Weiqin Chu, Shiqi Liu and Haijun Yu*
Chem. Commun., 2019, 55, 2138-2141
DOI: 10.1039/C8CC09677E, Communication
___________________________________________________________











 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at 
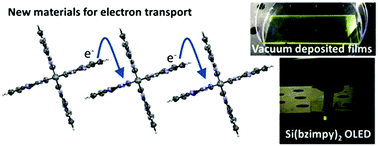
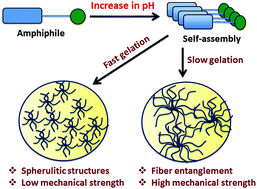
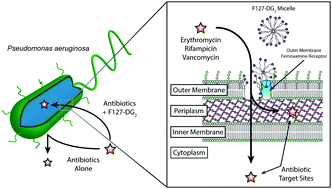
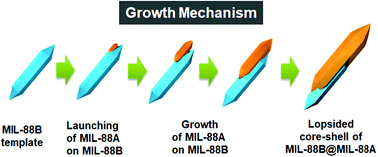

 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at 
 About the author
About the author