Organic compounds with conjugated electron structures are emerging as promising Li-ion battery cathodes due to their high capacity and environmental benignity. To make these cathodes practically feasible, organic electrodes are typically incorporated with metal ions to boost their energy densities. The addition of metal ions, however, usually jeopardizes the structural integrity of the electrodes and shortens battery lifetime.
Recently, three groups of Chinese researchers demonstrated that increasing the electrolyte concentration could effectively prolong the lifespan of metal-incorporated organic cathodes. The researchers studied cuprous tetracyano-quinodimethane (CuTCNQ), a Cu2+-containing organic Li-ion battery cathode, and observed its significantly improved cycling stability in a 7 M LiClO4 electrolyte compared to a 1 M electrolyte. This work was published recently in ChemComm.
CuTCNQ in a typical diluted electrolyte of 1 M LiClO4 exhibited unsatisfactory stability. Its first-cycle charging capacity reached ~180 mAh/g, but it dropped appreciably to 23 mAh/g after the first discharging process (Figure 1a). Concurrently, the electrolyte turned from clear to yellow (Figure 1b), due to the dissolution of TCNQ. These observations unequivocally showed the rapid disintegration of CuTCNQ in diluted electrolytes.
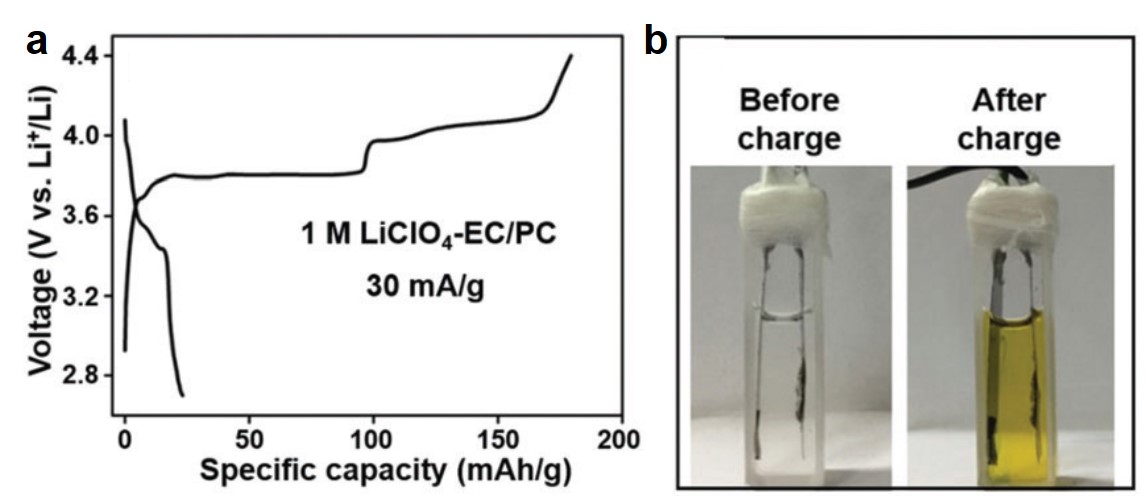
Figure 1. (a) The first-cycle charge-discharge profile of CuTCNQ in a liquid electrolyte containing ethylene carbonate (EC), propylene carbonate (PC) and 1 M LiClO4 (1 M LiClO4-EC/PC). (b) Photographs showing the electrolyte color before and after the first charge-discharge cycle.
CuTCNQ was found to be more stable in electrolytes with concentrations higher than 1 M. When the LiClO4 concentration increased to 3 M, 5 M and 7 M, the specific capacities of CuTCNQ retained after 50 consecutive charge-discharge cycles were ~25 mAh/g, ~70 mAh/g, and ~110 mAh/g, respectively (Figure 2a). All of these capacities were higher than that of CuTCNQ in 1 M LiClO4 after the same cycle number (<10 mAh/g). Additionally, the electrolytes experienced nearly no color change, suggesting little TCNQ was dissolved (Figure 2b).
The elevated stability of CuTCNQ correlates to the formation of Li+-ClO4– ion pairs in concentrated electrolytes (Figure 2c). With increasing LiClO4 concentration, Li+ and ClO4– tend to form ion pairs that coordinate with solvent molecules. Solvent-coordination reduces the number of free solvent molecules that can dissolve TCNQ, thus minimizing the dissolution of TCNQ.
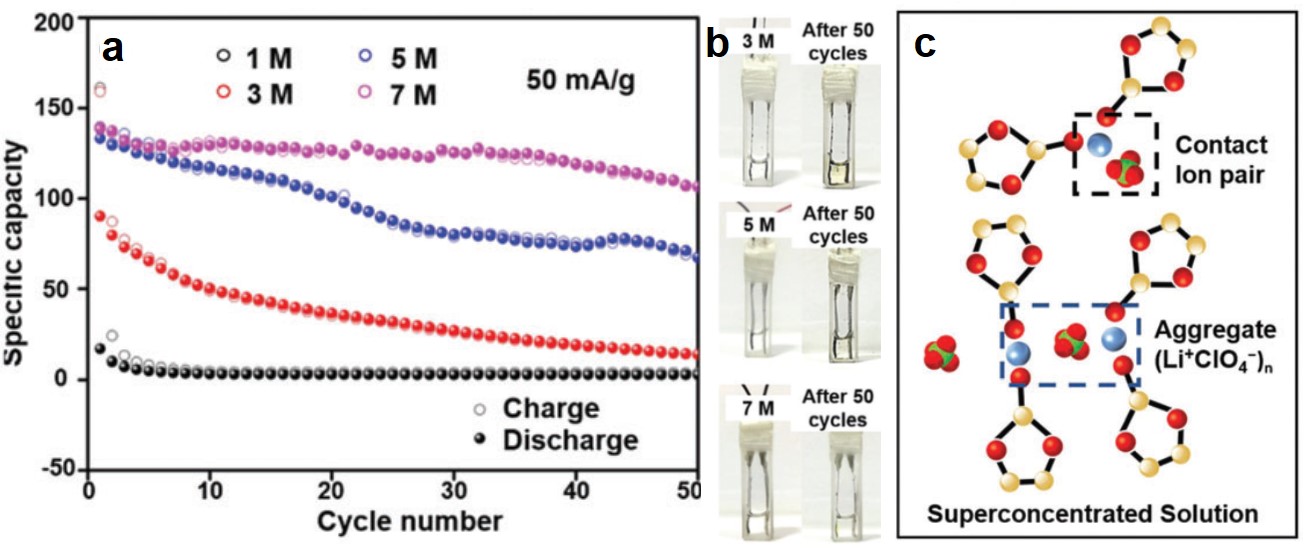
Figure 2. (a) The cycling stability performances of CuTCNQ with electrolytes with different LiClO4 concentrations. (b) Photographs showing the electrolyte color before and after 50 charge-discharge cycles at different LiClO4 concentrations. (c) Li+ and ClO4- form solvent-coordinated ion pairs in super-concentrated electrolytes (e.g., 7 M).
This work provides a facile approach to mitigate the capacity fading of CuTCNQ. The strategy may be extended to stabilize other metal-incorporated organic cathodes in Li-ion batteries.
To find out more please read:
Sustainable Cycling Enabled by A High-Concentration Electrolyte for Lithium-Organic Batteries
Ying Huang, Chun Fang, Wang Zhang, Qingju Liu and Yunhui Huang
Chem. Commun., 2019, 55, 608-611
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










