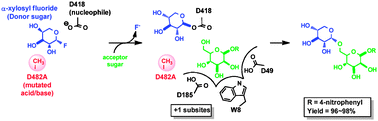
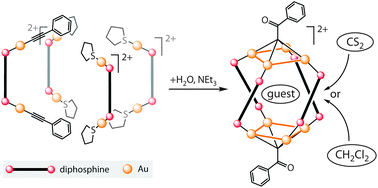
Simple aromatic compounds can self-assemble into low-dimensional aggregates with controlled architecture, according to US scientists.
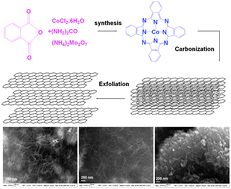
Yi Liu, at Lawrence Berkeley National Laboratory, and colleagues have shown that single molecule-thick two-dimensional nanosheets self-assemble from symmetric hexakis(alkoxy)triphenylene derivatives, and then further stack to give multilayered nanofibres.

Anisotropic nanostructures of organic semiconductors have good electronic and optical properties, making them suitable morphologies for advanced optoelectronic applications. But controlling the ordering of such materials at the molecular level remains a challenging task as it is affected by many structural and environmental aspects.
This simple solution process provides an attractive and convenient bottom-up path to hierarchical self-assembly nanostructures, Liu says.
For more information, read Liu’s ChemComm communication, free to access until 9th December.
If you are having trouble accessing free content in ChemComm, register for an RSC Publishing personal account today. To find out more, please visit the RSC Publishing Blog.