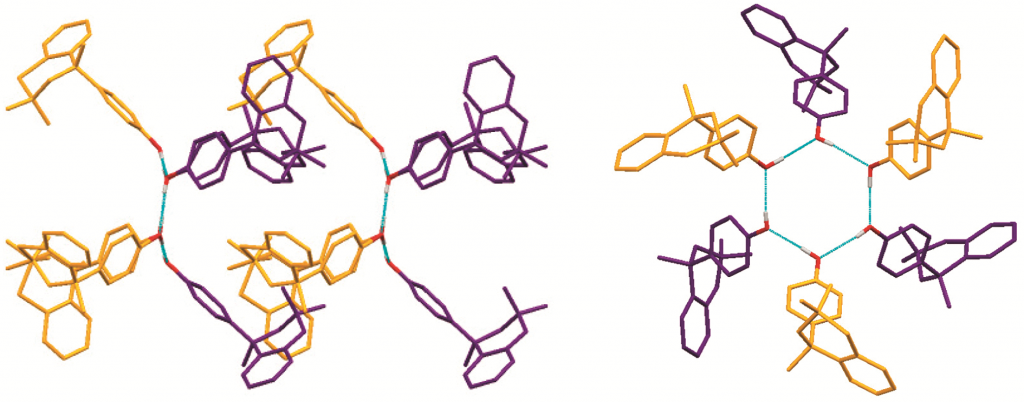
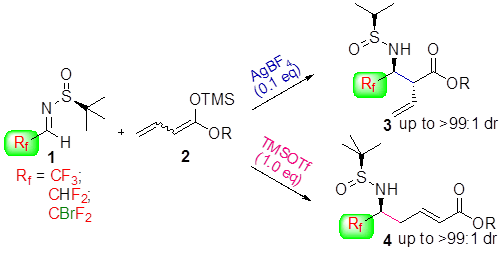
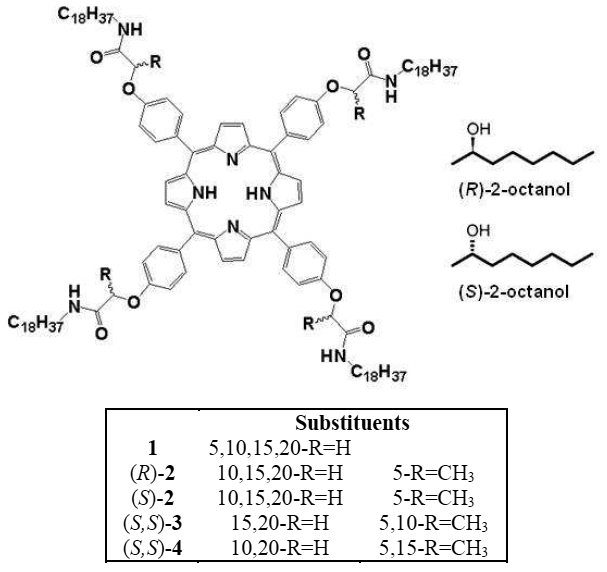
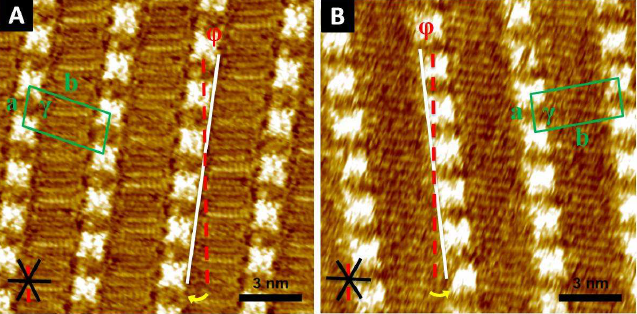
Scientists in Japan have shown that a dye can present more than five different colours according to the acidity of the solution it is in and can be used to visualise acid–base equilibria in non-polar solvents. It is extremely unusual for a single dye to demonstrate so many different colours.

Hydrogen bonding, deprotonation and different degrees of protonation all turn the dye, oxoporphyrinogen, a different colour. Hydrogen bonding of an anion to oxoporphyrinogen gives an increasingly blue colour depending on the strength of the interaction. Strongly basic substances cause oxoporphyrinogen to deprotonate and turn a pale brown hue. In the opposing direction, acids of certain strength can doubly protonate the dye. This induces tautomerisation of the dye’s structure, which varies its conjugated electronic form, leading to another significant colour change, in this case to red. Stronger acids quadruply protonate the dye, so all available electronegative atoms are protonated, turning it bright green.
Jonathan Hill, from the National Institute for Materials Science, Ibaraki, who led the work, says there are two main reasons to want to see acid–base equilibria in non-polar solvents…
Read the full article in Chemistry World
Read the original journal article in ChemComm:
Colorimetric visualization of acid–base equilibria in non-polar solvent
Atsuomi Shundo, Shinsuke Ishihara, Jan Labuta, Yosuke Onuma, Hideki Sakai, Masahiko Abe, Katsuhiko Ariga and Jonathan P. Hill
Chem. Commun., 2013, 49, 6870-6872
DOI: 10.1039/C3CC42859A