Bartosz Lewandowski’s ChemComm1st article ‘Chiral recognition of amino-acid esters by a glucose-derived macrocyclic receptor‘ is available now. We wanted to find out more about Bartosz and what it was like to reach this ChemComm Milestone in our interview below.
Read our with Bartosz interview here.
What are the main areas of research in your lab and what motivated you to take this direction?
The main topic which we are investigating is the use of monosaccharides as building blocks to create supramolecular receptors and assemblies. We want to take advantage of the intrinsic features of these biomolecules (e.g. water solubility, biocompatibility, modularity) and create new types of supramolecular systems and devices for controlled and selective encapsulation, transport and chemical transformations of molecular entities.
I was “hooked on sugars” during my Ph.D. studies in the group of Prof. Sławomir Jarosz where I explored the chemistry of sucrose. This was a great learning experience for me as I got to know the challenges associated with sugar chemistry, but was also able to appreciate the great potential of these biomolecules. And I felt that there are many exciting things that can be done with sugars, particularly in the context of supramolecular chemistry, which is exactly what we are working on right now.
Can you set this article in a wider context?
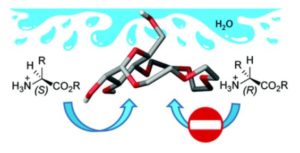
The ability to separate or detect enantiomers of bioactive molecules is of high importance since they very often have vastly different chemical and biological properties. Achieving this goal in aqueous media is particularly relevant if one wants to develop analytical tools for diagnostic or therapeutic purposes. Within our manuscript we demonstrated the efficacy of a simple glucose-based macrocycle for differentiation of amino-acid enantiomers in aqueous environments. Thus our results open up exciting opportunities for the development of molecular tools for chirality sensing and enantiomer separation of bioactive molecules.
What do you hope your lab can achieve in the coming year?
Firstly, I hope that we can build on the results we just published and develop further carbohydrate-based chiral receptors. We plan to utilize the modularity of monosaccharides and their potential for functional fine-tuning to create supramolecular receptors with additional attractive features (e.g. increased chemoselectivity, fluorescence). My other ambition for this and following years is to explore other research pathways with carbohydrate-based macrocycles and use them as building blocks to create novel functional supramolecular assemblies and perhaps even molecular machines.
Describe your journey to becoming an independent researcher.
I think the moment when I started to seriously consider becoming an academic researcher was when I joined the group of Prof. David Leigh for my post-doc. Designing and creating molecular machines is a tremendous scientific challenge. But for me it also contained an element of pure joy and excitement coming from assembling small molecular fragments piece-by-piece into a device that can perform complex tasks. And the satisfaction when the final goal was achieved rewarded all the difficulties and frustration that came along the way. And that’s when I thought “Yes, this is what I want to do in life.” That thought was then reinforced when I joined the group of Prof. Helma Wennemers. Working on highly multidisciplinary cutting-edge research and being immediately entrusted with supervision and guidance for junior co-workers (both students and Ph.D. students) allowed me to greatly mature as a scientist. It also inspired me to create my own research plan for the future. And at the end of 2015 I successfully applied for the position of a Senior Scientist in the Wennemers Group at the Laboratory of Organic Chemistry, ETH Zürich. This is a unique position which gives me the opportunity to build my independent research line while remaining an integral part of Prof. Wennemers’ team where we pursue exciting research projects.
What is the best piece of advice you have ever been given?
I’ve been very fortunate to have worked with many incredibly supportive people and I’ve received a lot of great advice from them. The two pieces that stuck with me the most over the years are:
“If you keep doing excellent work, good things will eventually come your way.” and “You should never talk yourself out of an experiment.”
Why did you choose to publish in ChemComm?
First of all because it’s one of the leading chemistry journals in the world with a broad impact on the scientific community. It’s also among my favourite journals to read when I screen recent literature. Finally, I was very keen on publishing my first independent work in ChemComm as this is where the most significant results of my PhD were published.
Read Bartosz’s ChemComm1st article and others in our collection ChemComm Milestones – First Independent Article. Follow @ChemCommun for all of the latest journal and #ChemCommMilestones news.