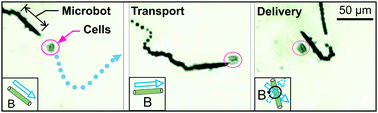
Microjet engines called microbots that can transport cells within a fluid to any desired location have been developed by scientists in Germany.
Manipulating nanomachines to transport biological matter in the body has been a challenge until now. Samuel Sanchez, from the Institute for Integrative Nanosciences in Dresden, and colleagues have shown that by using a magnet it is possible to navigate a microbot towards a specific cell within the body, pick it up from point A and transport it to point B.
The group made machines made up of hollow tubular structures containing a thin layer of platinum on the inside. They found that the machines moved independently in a peroxide solution when controlled externally by a small magnet manipulated with a joystick. The microbots can be directed towards suspended cells in solution, where they pick them up and transport them to the desired location. They released the cells from the tube by rapidly turning the magnet.
Sanchez and his team hope that in the future their microbots could perform visionary tasks within the body. “I would like to see our microbots swimming inside the bodies of animals, delivering drugs to required locations, for example, in the vicinity of cancer cells or replacing diseased cells with healthy ones,” he says.
For more information download Sanchez’s communication, where you can find videos of the swimming microbots in the ESI.











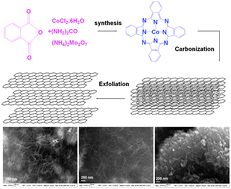
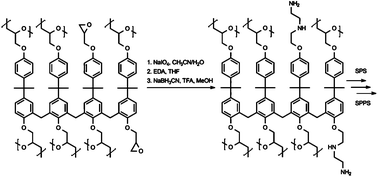



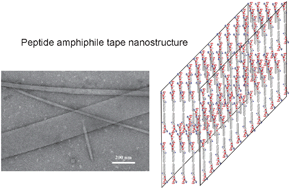
 We are delighted to invite nominations for the very first ChemComm Emerging Investigator Lectureship. The lectureship, which will be awarded annually, will recognise an emerging scientist in the early stages of their independent academic career.
We are delighted to invite nominations for the very first ChemComm Emerging Investigator Lectureship. The lectureship, which will be awarded annually, will recognise an emerging scientist in the early stages of their independent academic career.