Hydrogen gas is a zero-emission energy resource promising to replace diminishing fossil fuels. The electrolysis of water is a sustainable way to acquire hydrogen gas, but this non-spontaneous process demands electricity to proceed. Therefore, hydrogen evolution reaction (HER) catalysts are used to reduce the energy cost or overpotential of the electrolysis.
Researchers are pursuing ultrafine nanoparticles as HER catalysts due to their high catalytic activity. For example, the HER catalytic activities of Ru nanoparticles are reportedly 100-200% higher than those of bulk Ru catalysts. Unfortunately, the preparation of well-dispersed nanoparticles is challenging because nanoparticles are prone to aggregate together.
Recently in ChemComm, Fuqiang Chu, Yong Qin and coworkers from Changzhou University, China addressed the challenge. They utilized a Ru-based coordination complex and cyanuric acid as the reactants, and synthesized high-performance HER catalysts composed of ~2 nm Ru nanoparticles uniformly dispersed on graphene sheets. During the thermal annealing step in the synthesis, the ligands of the complex and the cyanuric acid both decompose to nitrogen-doped carbon shells covering the as-formed Ru nanoparticles. These shells serve as spacers that prevent particle aggregation (Figure 1).
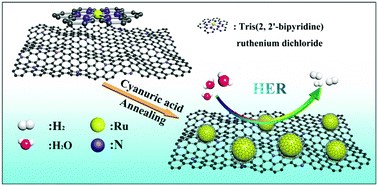
Figure 1. An illustration of the synthesis of carbon-coated Ru ultrafine nanoparticles on graphene sheets. Tris(2,2′-bipyrindine) ruthenium dichloride is the precursor of the Ru nanoparticles.
In both the acidic and the alkaline electrolytes, the 2 nm Ru particles (RuNC-2) display lower overpotentials and higher current densities than the 5 nm Ru particles (Figure 2) without the carbon coating (RuNC-5). Remarkably, the 2 nm particles showed comparable performance to the benchmark Pt catalyst in the acidic electrolyte (the red and black curves in Figure 2a).

Figure 2. Linear sweep voltammograms of ~3 nm Pt nanoparticles (PtNC), 2 nm Ru nanoparticles (RuNC-2) and 5 nm Ru nanoparticles (RuNC-5) in (a) 0.5 M H2SO4 and (b) 1 M KOH aqueous solutions.
The concept of the in-situ generation of protective coatings could inspire the synthesis of other ultra-small nanoparticles to potentially push the HER catalytic performance to new heights.
To find out more please read:
Yutong Li, Fuqiang Chu, Yang Liu, Yong Kong, Yongxin Tao, Yongxin Li and Yong Qin
Chem. Commun., 2018, DOI: 10.1039/c8cc08276f
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










