Nominations are now open for the Cram Lehn Pedersen Prize in Supramolecular Chemistry. The prize, sponsored by ChemComm, is organised by the committee of the International Symposium on Macrocyclic and Supramolecular Chemistry and is awarded each year to a young supramolecular chemist.
The Cram Lehn Pedersen Prize is named in honour of the winners of the 1987 Nobel Prize in Chemistry and recognizes significant original and independent work in supramolecular chemistry. Previous winners include Oren Schermann, Tomoki Ogoshi, and Jonathan Nitschke.
The Prize
The winner will receive:
- £2000
- free registration for the ISMSC meeting in Strasbourg, France
- the opportunity to give a lecture at the ISMSC as well as undertake a short lecture tour after the meeting, in consultation with the Editor of ChemComm
Eligibility
To be eligible for the award you must be within 10 years of receiving your PhD on 31st December 2014
Nomination Instructions
You may nominate yourself or someone else. Please send CV, list of publications (divided into publications from PhD and postdoc and publications from independent work), and, if desired, a letter of support to Prof. Roger Harrison (ISMSC Secretary) at rgharris@chem.byu.edu by 31st December 2014












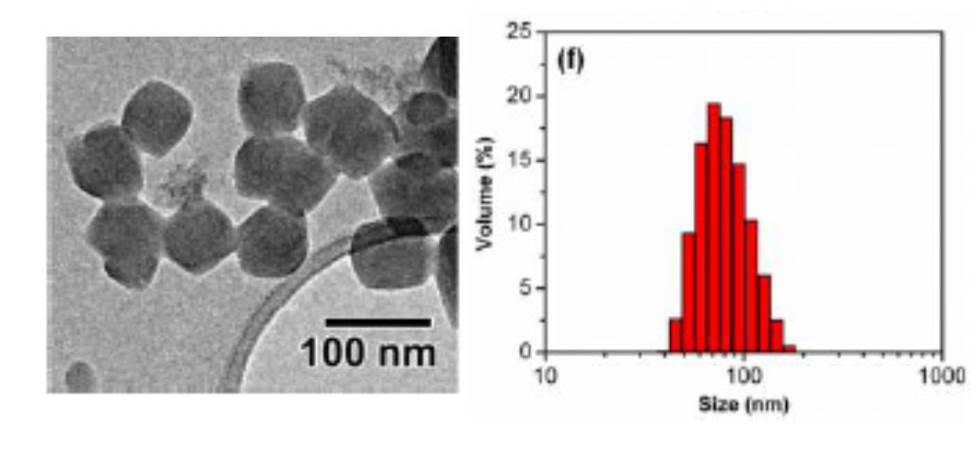


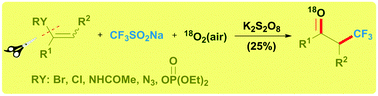
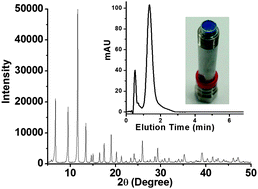
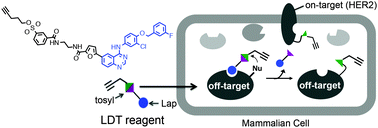
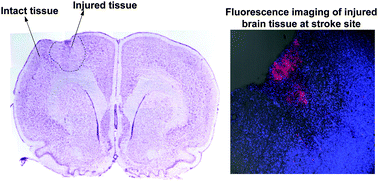
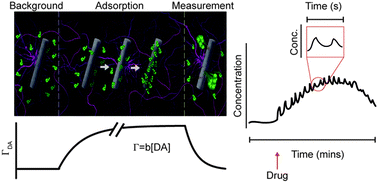
 Covalency, a term describing bonding by sharing electrons, divides opinion when mentioned alongside hydrogen bonding. Worried that the concept of hydrogen bonding has been getting fuzzier over time, scientists in Germany have sought
Covalency, a term describing bonding by sharing electrons, divides opinion when mentioned alongside hydrogen bonding. Worried that the concept of hydrogen bonding has been getting fuzzier over time, scientists in Germany have sought