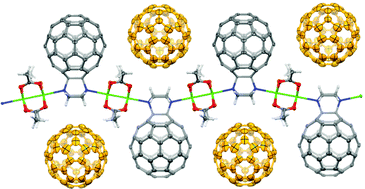
Since its structural realisation in 1985, C60 has garnered much attention in the chemical world for not only its spherical shape, but also its stability, electronic properties and the ability to do chemistry on its surface.
One such avenue that has proven popular in recent times is the incorporation of C60 into one-, two- and three-dimensional arrays, either covalently or non-covalently, in attempts to control the distribution of the molecules in the solid- or solution-phase. One problem that arises in the synthesis of these extended frameworks, however, is that there often a large amount of disorder and void space in the structure, so it can be difficult to ascertain with much degree of certainty how these C60 molecules are oriented. This uncertainty can consequentially result in the properties and behaviours of the new materials remaining unidentified.
Now, researchers from the University of California, Davis – Marilyn Olmstead and Alan Balch – have shown that coordination chemistry can be used to not only generate polymers that covalently link molecules of functionalised C60 in such a manner that can they can be studied crystallographically, but also that these polymers can be used to capture free C60 and C70.
Initially, polymers of C60 were synthesised through the mono-functionalisation of C60 with a piperazyl group, which, on account of its two tertiary amines, can coordinate in a linear fashion with transition metal ions, in this case rhodium(II) acetate. Upon the combination of these two components, a linear one-dimensional polymer was formed, in which it could be seen crystallographically that the C60 moieties were positioned on alternating sides of the polymer chain. These polymer chains were further found to extend into two dimensions through the interdigitation of neighbouring chains in a zipper-like fashion.
Perhaps more interestingly is that when these polymer chains were synthesised in the presence of either C60 or C70, free molecules of C60 or C70 were seen to occupy the void spaces between the C60 molecules of the polymer. Additionally, if a mixture of C60 and C70 was present in the polymer synthesis, it was observed that only C60 was captured by the polymer, most likely as a result of a better geometric match between the polymer and the spherical C60 in preference to the more elongated shape of C70.
This work elegantly demonstrates the generation of not only a self-assembling C60-containing polymer that can be characterised structurally in the solid state, but of one that can entrap free molecules of C60 selectively over molecules of C70. Based on the properties of free C60 and transition metal complexes, the electronic and chromophoric properties of such a crystalline system could also be expected to offer some noteworthy results.
Read this HOT ChemComm article in full!
Zipping up fullerenes into polymers using rhodium(II) acetate dimer and N(CH2CH2)2NC60 as building blocks
Amineh Aghabali, Marilyn M. Olmstead and Alan L. Balch
Chem. Commun., 2014, Advance Article.
DOI: 10.1039/C4CC06995A
Biography
 Anthea Blackburn is a guest web writer for Chemical Communications. Anthea is a graduate student hailing from New Zealand, studying at Northwestern University in the US under the tutelage of Prof. Fraser Stoddart (a Scot), where she is exploiting supramolecular chemistry to develop multidimensional systems and study the emergent properties that arise in these superstructures. When time and money allow, she is ambitiously attempting to visit all 50 US states before graduation.
Anthea Blackburn is a guest web writer for Chemical Communications. Anthea is a graduate student hailing from New Zealand, studying at Northwestern University in the US under the tutelage of Prof. Fraser Stoddart (a Scot), where she is exploiting supramolecular chemistry to develop multidimensional systems and study the emergent properties that arise in these superstructures. When time and money allow, she is ambitiously attempting to visit all 50 US states before graduation.