US scientists have found a way to stop solid byproducts clogging channels in continuous flow reactors, a problem that has hampered their progress for use in manufacturing pharmaceuticals.
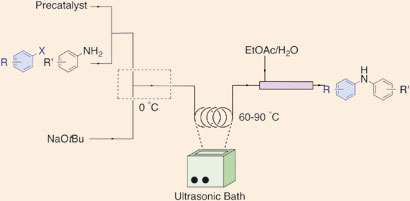
Klavs Jensen, Stephen Buchwald and their team at the Massachusetts Institute of Technology believe that flow methods will become increasingly important in the future of pharmaceuticals and chemical manufacturing. ‘One of the biggest hurdles is handling solids,’ says group member Timothy Noël. ‘Precipitates can form during the reactions, which usually lead to irreversible clogging of microchannels in the reactors.’ Previous methods suggested to overcome this problem include introducing another solvent to dissolve the solids, but this can reduce the overall efficiency of the reactions. Now, the team have used an ultrasound bath to break up the byproducts to prevent clogging.
Traditionally, pharmaceutical manufacture is done in a batch-based system, but the process suffers from interruptions and the need to transport material between batch reactors. Performing these reactions in a continuous flow system would speed up the process and reduce chemical waste.
The team tested the method on palladium-catalysed C-N cross-coupling reactions, making amines that are common in biologically active molecules. The reactions couple aryl halides to nitrogen nucleophiles and form byproducts – inorganic salts – that are insoluble in the solvents used.
As a result, says Noël, they were able to obtain diarylamine products with reaction times ranging from 20 seconds to 10 minutes. At very short residence times (time in the reactor under reaction conditions) they observed a significantly higher rate for the reaction in flow compared to the equivalent batch experiments. With high conversions in short reaction times, they were able to reduce the catalyst loading in flow to just 0.1 mol per cent. ‘Extremely low catalyst loadings such as these are of particular interest to the pharmaceutical industry,’ says Noël.
Noël believes that in the future microfluidics will be used to construct increasingly complex molecules. Different devices will automate and integrate many synthetic steps that are currently performed using the more traditional and time-consuming batch-based practices.
Oliver Kappe, from the Christian Doppler Laboratory for Microwave Chemistry, Institute of Chemistry, Karl-Franzens-University Graz says: ‘Jensen and Buchwald clearly demonstrate that immersing a flow device into an ultrasound bath can prevent clogging problems that unfortunately are all too familiar to the flow/microreactor community.’
Sarah Corcoran
Find out more by downloading the Chemical Science Edge article.
Stephen Buchwald is Associate Editor for Chemical Science. Submit your exceptional organic research to him today to be seen with the best.
Also of interest:
Continuous flow multi-step organic synthesis
Damien Webb and Timothy F. Jamison
Chem. Sci., 2010, 1, 675-680, DOI: 10.1039/C0SC00381F, Minireview
Comments Off on Unclogging the problems of flow chemistry