A water molecule can act as a spy to sense and expose the reasons behind the anomeric effect in carbohydrates.
Since it was identified more than 50 years ago, the anomeric effect’s origins have been hotly debated. Scientists have found it difficult to separate stereoelectronic effects from other potential influences, including solvation.
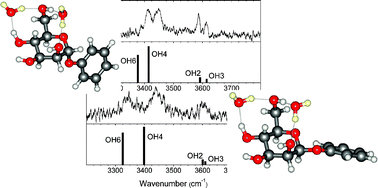
Using vibrational spectroscopy, researchers have studied doubly hydrated anomers of a mannopyranoside under molecular beam conditions in the gas phase. By substituting heavy water (D2O) for H2O, they separated the carbohydrate (OH) bands from the water (OD) bands, helping them to interpret differences in the anomers’ vibrational signatures. One of the water molecules acted as a remarkably sensitive reporter, able to sense and expose subtle stereoelectronic changes through the resulting changes in its hydrogen-bonded interaction with the substrate.
Eager to read more? Download the full Edge article in Chemical Science for free:
Heavy water hydration of mannose: the anomeric effect in solvation, laid bare
Nitzan Mayorkas, Svemir Rudić, Benjamin G. Davis and John P. Simons, Chem. Sci., 2011, DOI: 10.1039/C1SC00002K