This month sees the following articles in Chemical Science that are in the top ten most accessed:-
A synthesis of strychnine by a longest linear sequence of six steps
David B. C. Martin and Christopher D. Vanderwal
Chem. Sci., 2011, 2, 649-651, DOI: 10.1039/C1SC00009H, Edge Article
Hydroxyl-directed C-H carbonylation enabled by mono-N-protected amino acid ligands: An expedient route to 1-isochromanones
Yi Lu, Dasheng Leow, Xisheng Wang, Keary M. Engle and Jin-Quan Yu
Chem. Sci., 2011, 2, 967-971, DOI: 10.1039/C0SC00633E, Edge Article
Benzoquinone-derived sulfinyl imines as versatile intermediates for alkaloid synthesis: Total synthesis of (-)-3-demethoxyerythratidinone
Kangway V. Chuang, Raul Navarro and Sarah E. Reisman
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C1SC00095K, Edge Article
Transition metal-catalyzed cross coupling with N-acyliminium ions derived from quinolines and isoquinolines
Thomas J. A. Graham, Jason D. Shields and Abigail G. Doyle
Chem. Sci., 2011, 2, 980-984, DOI: 10.1039/C1SC00026H, Edge Article
Asymmetric catalytic reactions by NbO-type chiral metal-organic frameworks
Kyung Seok Jeong, Yong Bok Go, Sung Min Shin, Suk Joong Lee, Jaheon Kim, Omar M. Yaghi and Nakcheol Jeong
Chem. Sci., 2011, 2, 877-882, DOI: 10.1039/C0SC00582G, Edge Article
Catalytic, enantioselective synthesis of stilbene cis-diamines: A concise preparation of (-)-Nutlin-3, a potent p53/MDM2 inhibitor
Tyler A. Davis and Jeffrey N. Johnston
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C1SC00061F, Edge Article
Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide
David S. Surry and Stephen L. Buchwald
Chem. Sci., 2011, 2, 27-50, DOI: 10.1039/C0SC00331J, Perspective
Optimization of distyryl-Bodipy chromophores for efficient panchromatic sensitization in dye sensitized solar cells
Safacan Kolemen, O. Altan Bozdemir, Yusuf Cakmak, Gokhan Barin, Sule Erten-Ela, Magdalena Marszalek, Jun-Ho Yum, Shaik M. Zakeeruddin, Mohammad K. Nazeeruddin, Michael Grätzel and Engin U. Akkaya
Chem. Sci., 2011, 2, 949-954, DOI: 10.1039/C0SC00649A, Edge Article
A quantum-chemical perspective into low optical-gap polymers for highly-efficient organic solar cells
Chad Risko, Michael D. McGehee and Jean-Luc Brédas
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C0SC00642D, Perspective
Cation-induced molecular motion of spring-like [2]catenanes
Alexandre V. Leontiev, Christopher J. Serpell, Nicholas G. White and Paul D. Beer
Chem. Sci., 2011, 2, 922-927, DOI: 10.1039/C1SC00034A, Edge Article
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Chemical Science? Then why not contact us today with your suggestions.
Comments Off on Top ten most accessed articles in March


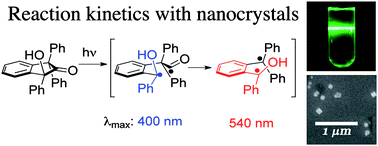









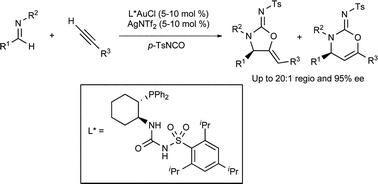
 On Monday I attended the
On Monday I attended the 


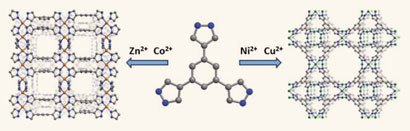


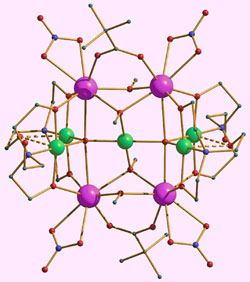 Scientists have laid the foundations for a high-performance ‘molecular fridge’ capable of reaching temperatures within a few thousandths of a degree of absolute zero (0K) with a high degree of efficiency. Such ultracoolers could have applications in areas such as ultra-low temperature physics, where alternative technologies such as those that rely on expensive and rare helium-3 could be unsuitable or too costly.
Scientists have laid the foundations for a high-performance ‘molecular fridge’ capable of reaching temperatures within a few thousandths of a degree of absolute zero (0K) with a high degree of efficiency. Such ultracoolers could have applications in areas such as ultra-low temperature physics, where alternative technologies such as those that rely on expensive and rare helium-3 could be unsuitable or too costly.