This month sees the following articles in Chemical Science that are in the top ten most accessed:-
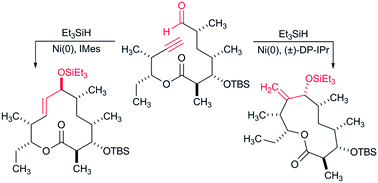
General palladium-catalyzed aerobic dehydrogenation to generate double bonds
Weiming Gao, Zhiqi He, Yong Qian, Jing Zhao and Yong Huang
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00661D
Direct aerobic a,ß-dehydrogenation of aldehydes and ketones with a Pd(TFA)2/4,5-diazafluorenone catalyst
Tianning Diao, Tyler J. Wadzinski and Shannon S. Stahl
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00724F
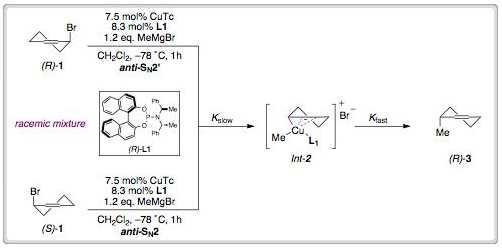
Catalytic enantioselective electrocyclic cascades
Eleanor E. Maciver, Peter C. Knipe, Andrew P. Cridland, Amber L. Thompson and Martin D. Smi
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00697E
Highly stereoselective catalytic conjugate addition of acyl anion equivalent to nitroolefins
Daisuke Uraguchi, Yusuke Ueki and Takashi Ooi
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00678A
Rollover’ cyclometalation – early history, recent developments, mechanistic insights and application aspects
Burkhard Butschke and Helmut Schwarz
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00651G
Bifunctional organo/metal cooperative catalysis with cinchona alkaloid scaffolds
Linus Stegbauer, Filippo Sladojevich and Darren J. Dixon
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00416F
Organocatalytic enantioselective construction of nitrocyclohexanes containing multiple chiral centres via a cascade reaction
Sundaram Rajkumar, Kenneth Shankland, Geoffrey D. Brown and Alexander J. A. Cobb
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00592H
Aggregation-induced emission in BF2-hydrazone (BODIHY) complexes
Yin Yang, Xin Su, Calden N. Carroll and Ivan Aprahamian
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00658D
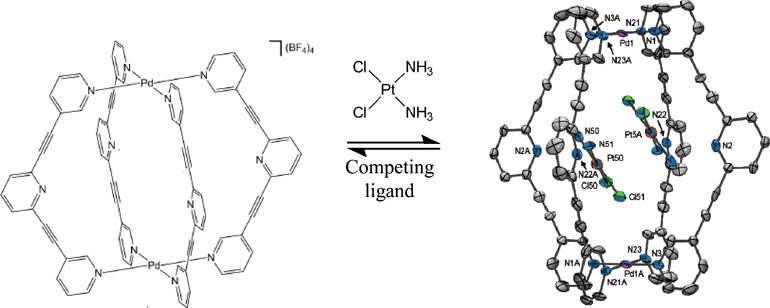
The first group 4 metal bis(imido) and tris(imido) complexes
Andrew D. Schwarz, Alastair J. Nielson, Nikolas Kaltsoyannis and Philip Mountford
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00786F
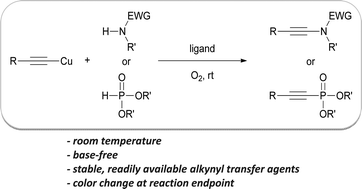
Copper-catalyzed direct oxidative synthesis of a-ketoamides from aryl methyl ketones, amines, and molecular oxygen
Feng-Tian Du and Jian-Xin Ji
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C1SC00312G
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Chemical Science? Then why not submit to us today or alternatively contact us with your suggestions.
Comments Off on Top ten most accessed articles in November