This exciting article just published in Chemical Science by Professor W.E. Moerner and colleagues describes a novel method of introducing fluorophores of single-molecule quality into live bacterial cells for super-resolution imaging studies.
Single-molecule fluorescence imaging works by converting a dark fluorogen into a bright emitter. The really interesting aspect of this current study is that this conversion is achieved enzymatically. They have synthesised a nitro-aryl fluorogen (dark fluorogen) which is converted by a nitroreductase enzyme into a push-pull red-emitting fluorophore (bright emitter).
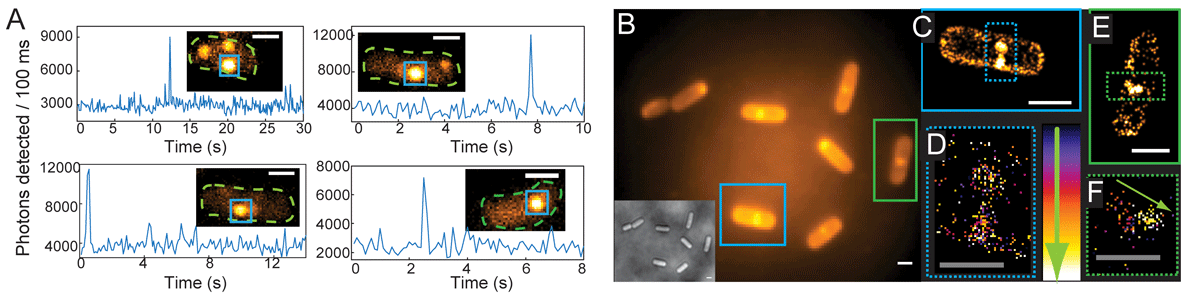
This new method allows the neutral dye molecule to enter the cell in a fluorescently ‘deactivated’ state. The substrate is then photoactivated by reaction with the enzyme, producing the fluorescent products – which is bright and detectable on the single-molecule level. The concentration can also be controlled by the level of substrate uptake – providing the opportunity for a variety of novel labeling systems.
The authors also present a detailed characterization of the spectral and photophysical properties of the fluorescent product, as well as the enzymatic kinetics in vitro.
Read the full article:
Enzymatic Activation of Nitro-Aryl Fluorogens in Live Bacterial Cells for Enzymatic Turnover-Activated Localization Microscopy
Marissa K. Lee, Jarrod Williams, Robert Twieg, Jianghong Rao and W.E. Moerner
Chem. Sci., 2012, DOI: 10.1039/C2SC21074F
Take a look at the high-quality physical chemistry research recently published in Chemical Science:
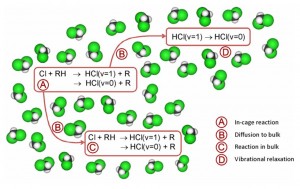
Vibrationally resolved dynamics of the reaction of Cl atoms with 2,3-dimethylbut-2-ene in chlorinated solvents
Fawzi Abou-Chahine, Stuart Greaves, Greg Dunning, Andrew Orr-Ewing, Gregory M Greetham, Ian P Clark and Michael Towrie
Chem. Sci., 2012, DOI: 10.1039/C2SC21267F
Nanomechanical properties of molecular-scale bridges as visualised by intramolecular electronic energy transfer
Anthony Harriman, Effat Bahaidarah, Raymond Ziessel, Mohammed Alamiry and Delphine Hablot
Chem. Sci., 2012, DOI: 10.1039/C2SC21505E
Role of conformational structures and torsional anharmonicity in controlling chemical reaction rates and relative yields: butanal + HO2 reactions
Jingjing Zheng, Prasenjit Seal and Donald G. Truhlar
Chem. Sci., 2013, DOI: 10.1039/C2SC21090H
Tridentate Cobalt complexes as alternate redox couples for high efficiency Dye Sensitized Solar Cells
Ben Aribia Kais, Thomas Moehl, Shaik M Zakeeruddin and Michael Gratzel
Chem. Sci., 2012, DOI: 10.1039/C2SC21401F
Cu2O|NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting
Chia-Yu Lin, Yi-Hsuan Lai, Dirk Mersch and Erwin Reisner
Chem. Sci., 2012, DOI: 10.1039/C2SC20874A
Perspective
Impurities in graphenes and carbon nanotubes and their influence on the redox properties
Martin Pumera, Adriano Ambrosi and Elaine Lay Khim Chng
Chem. Sci., 2012, DOI: 10.1039/C2SC21374E
Sign-up to our free contents e-alert or newsletter to receive the latest physical chemistry articles from Chemical Science.
Comments Off on Super-resolution single-molecule imaging