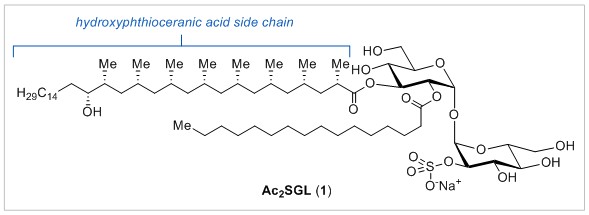
US scientists have found a way to control the direction of microscopic swimming robots using lasers. This is the first time that anyone has used a method like this to control a microscale particle in solution, they say.
Haw Yang at Princeton University and his colleagues used a polystyrene particle, with one half of its sphere coated in gold, as the swimmer. This type of particle is known as a Janus particle as it has two ‘faces’ that show different physical properties. The team used these properties to manipulate its direction. The Janus particle moves randomly in a liquid via Brownian motion, and when the particle is facing the right direction, the team uses a laser to give it a nudge forward.

The microswimmer is nudged towards a target by laser when it's facing the right direction
The researchers found that the laser propels the Janus particle by heating up the gold half of the sphere, which propels it forward. The polystyrene half doesn’t react to the laser as the laser essentially passes through it. The team used short bursts of the laser, and if the particle went off target, they stopped nudging it until it came back round to the right position.
Read the full article in Chemistry World
Read the original journal article:
Harnessing thermal fluctuations for purposeful activities: the manipulation of single micro-swimmers by adaptive photon nudging
Bian Qian, Daniel Montiel, Andreas Bregulla, Frank Cichos and Haw Yang
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C2SC21263C